RiboFree library prep
RiboFree library prep test for Mcap developmental time series Hawaii 2023
This post details the info about the library prep steps for the M. capitata developmental time series experiment in Hawaii 2023. The github for that project is linked here. I’m using the Zymo-Seq RiboFree Total RNA Library Kit for total RNA library prep (I got the 12 prep kit). See Zymo’s protocol here.
After sequencing n=1 from each time point from the Mcap 2023 project, we decided to move forward with sequencing n=3 from each time point in the ambient. Today, I used samples M8, M14, M28, M39, M48, M51, M61, M63, M74, M75, M87, and M88. M8, M14, M28, M48, M51 were extracted on 2/22/24 and M39, M61, M63, M74, M75, M87, and M88 were extracted on 5/2/24.
The kit needs a minimum input of 10 of total RNA or a maximum input of 250 ng of total RNA. I’m used 190 ng as my input concentration; Maggie did a similar input for her ribofree preps). Here’s the breakdown of input RNA volumes for each sample:
| TubeID | Qubit RNA Average (ng/uL) | RNA (uL) | DNA/RNA free water (uL) | Total starting volume (uL) | Primer |
|---|---|---|---|---|---|
| M8 | 39.2 | 4.8 | 3.2 | 8 | 36 |
| M14 | 115 | 1.7 | 6.3 | 8 | 37 |
| M28 | 31.8 | 6.0 | 2.0 | 8 | 38 |
| M39 | 21.3 | 8.9 | -0.9 | 8 | 39 |
| M48 | 114 | 1.7 | 6.3 | 8 | 40 |
| M51 | 31.5 | 6.0 | 2.0 | 8 | 41 |
| M61 | 29.8 | 6.4 | 1.6 | 8 | 42 |
| M63 | 29.8 | 6.4 | 1.6 | 8 | 43 |
| M74 | 32.4 | 5.9 | 2.1 | 8 | 44 |
| M75 | 27.4 | 6.9 | 1.1 | 8 | 45 |
| M87 | 71.6 | 2.7 | 5.3 | 8 | 46 |
| M88 | 97 | 2.0 | 6.0 | 8 | 47 |
Here’s the RiboFree library prep workflow:
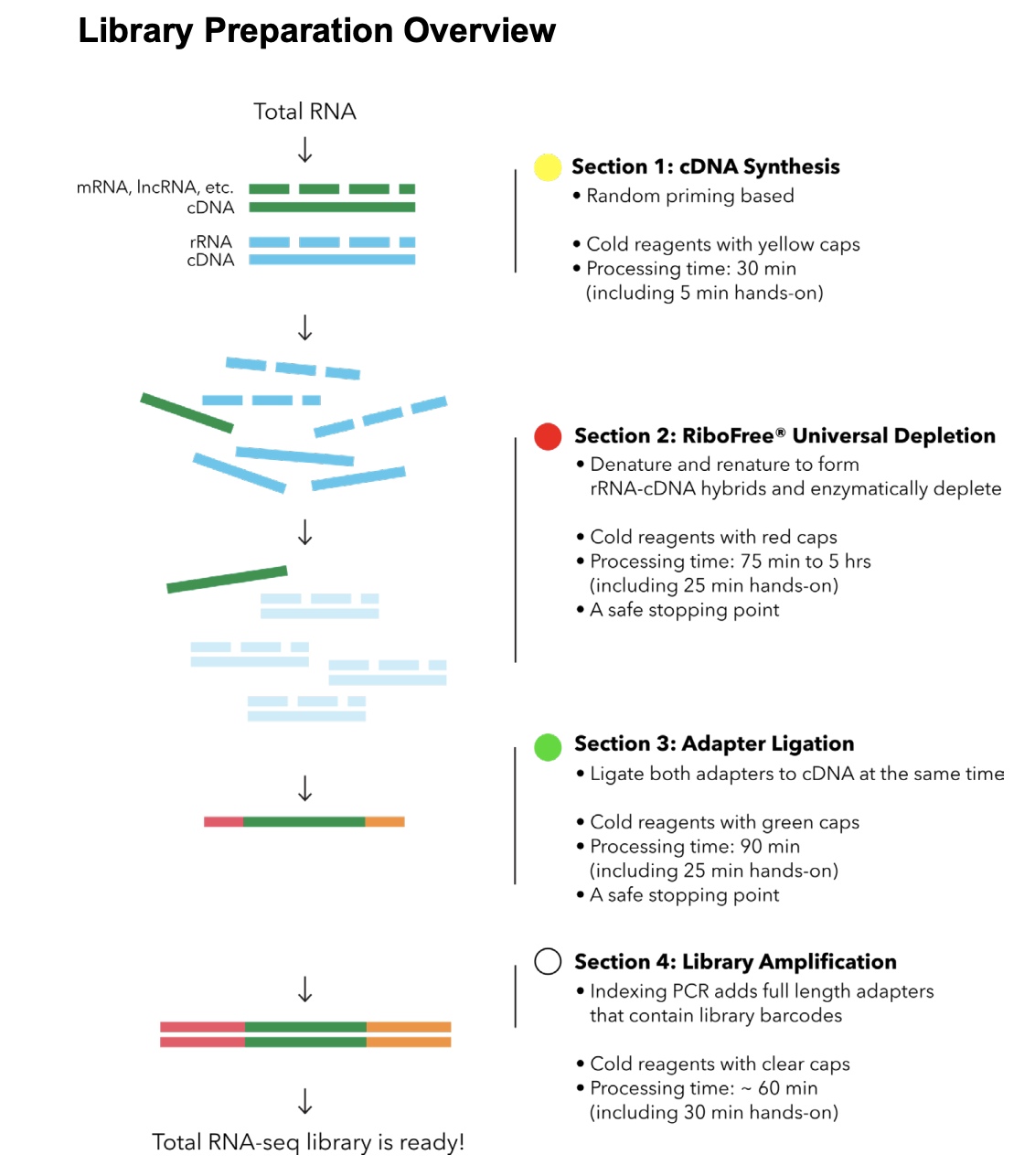
Materials
- Zymo-Seq RiboFree library kit
- PCR tubes
- Thermocycler
- Heating block
- Mini centrifuge
- Vortex
- Aluminum beads (to keep things on ice)
- Magnetic stand for PCR tubes
- 1.5 mL tubes
- Kit contents
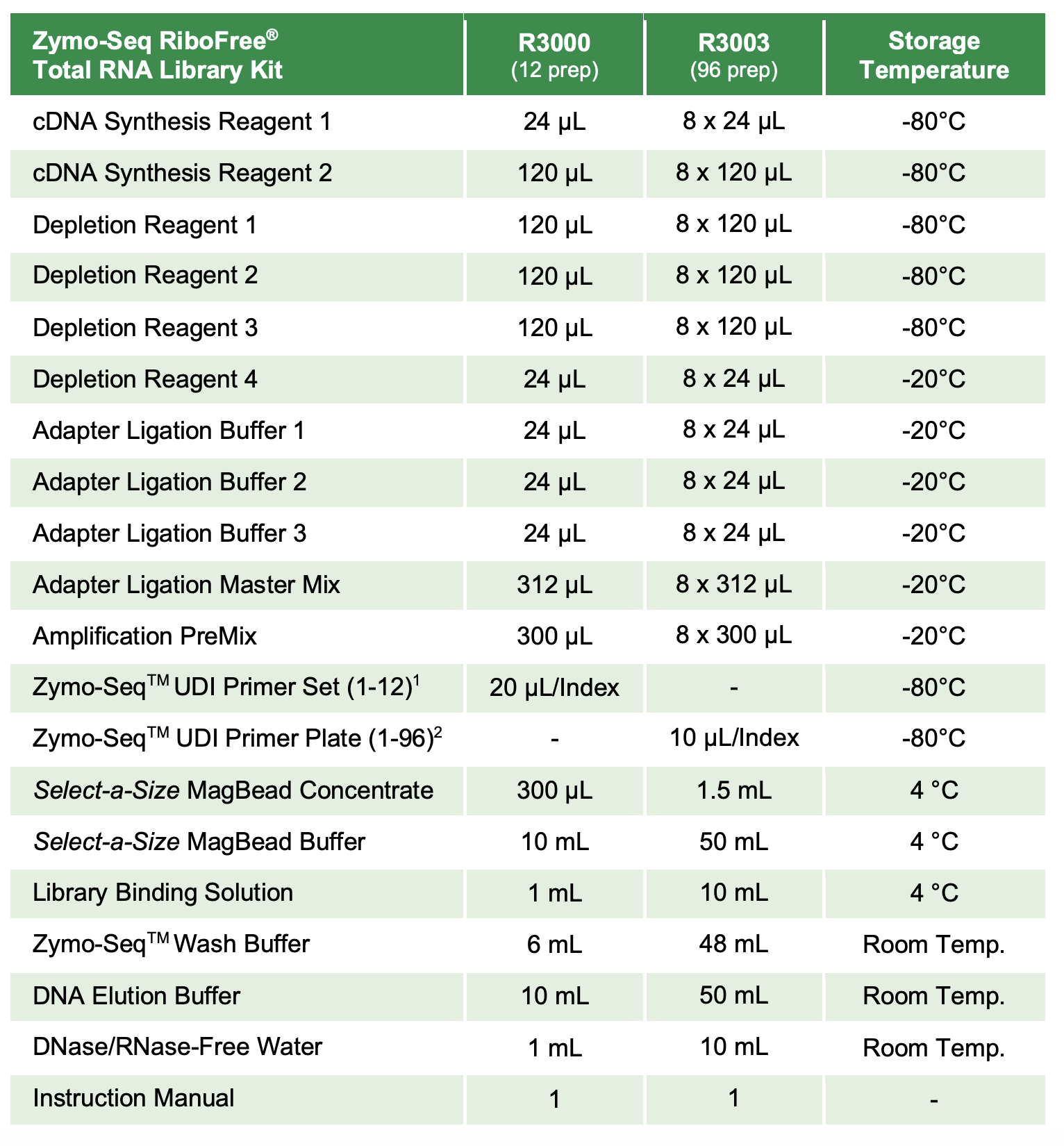
Protocol
Protocol was followed according to this post. The only difference is that the number of PCR library amplification cycles was changed to 15 from 11.
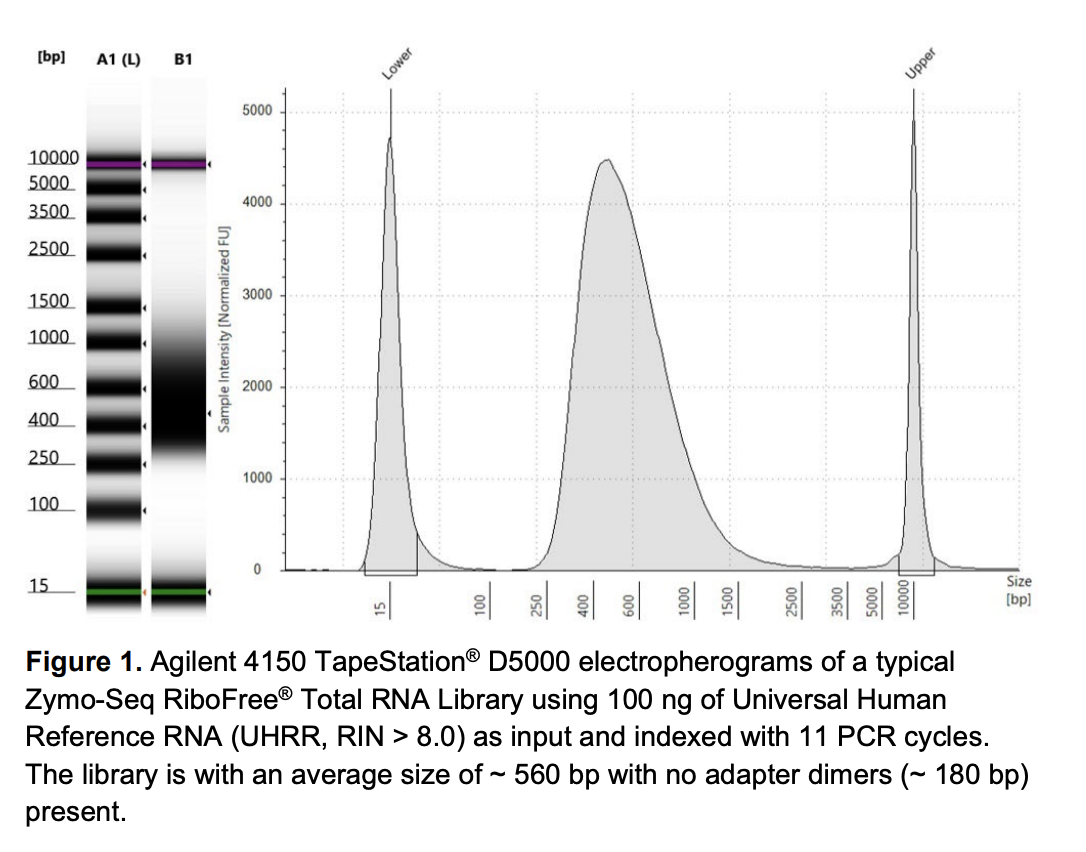
QC
Run DNA Tapestation for visualize libraries. Here’s an example of what the library should look like on a Tapestation:
Here’s how my samples turned out on 5/27/24. See full report here.
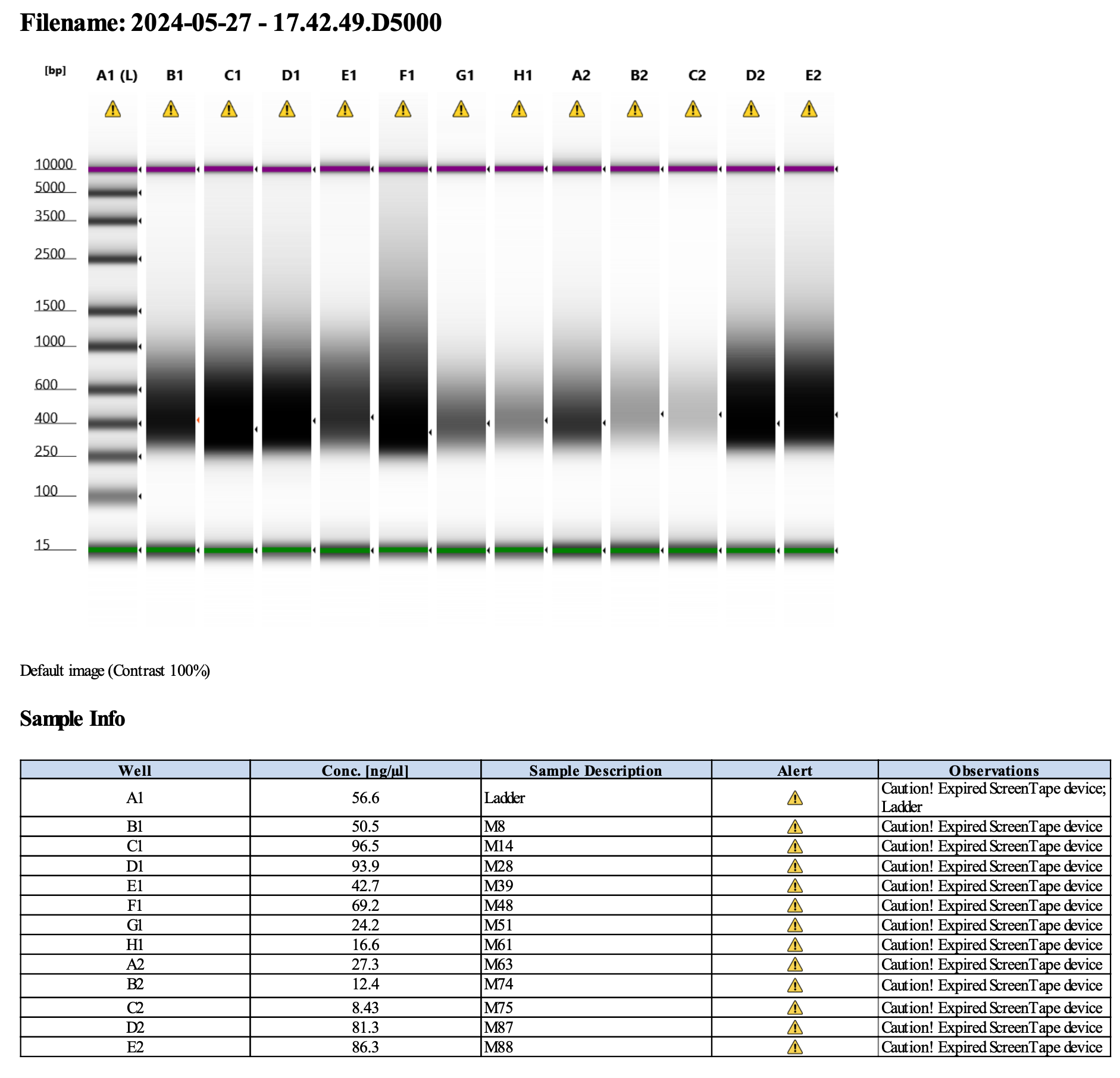
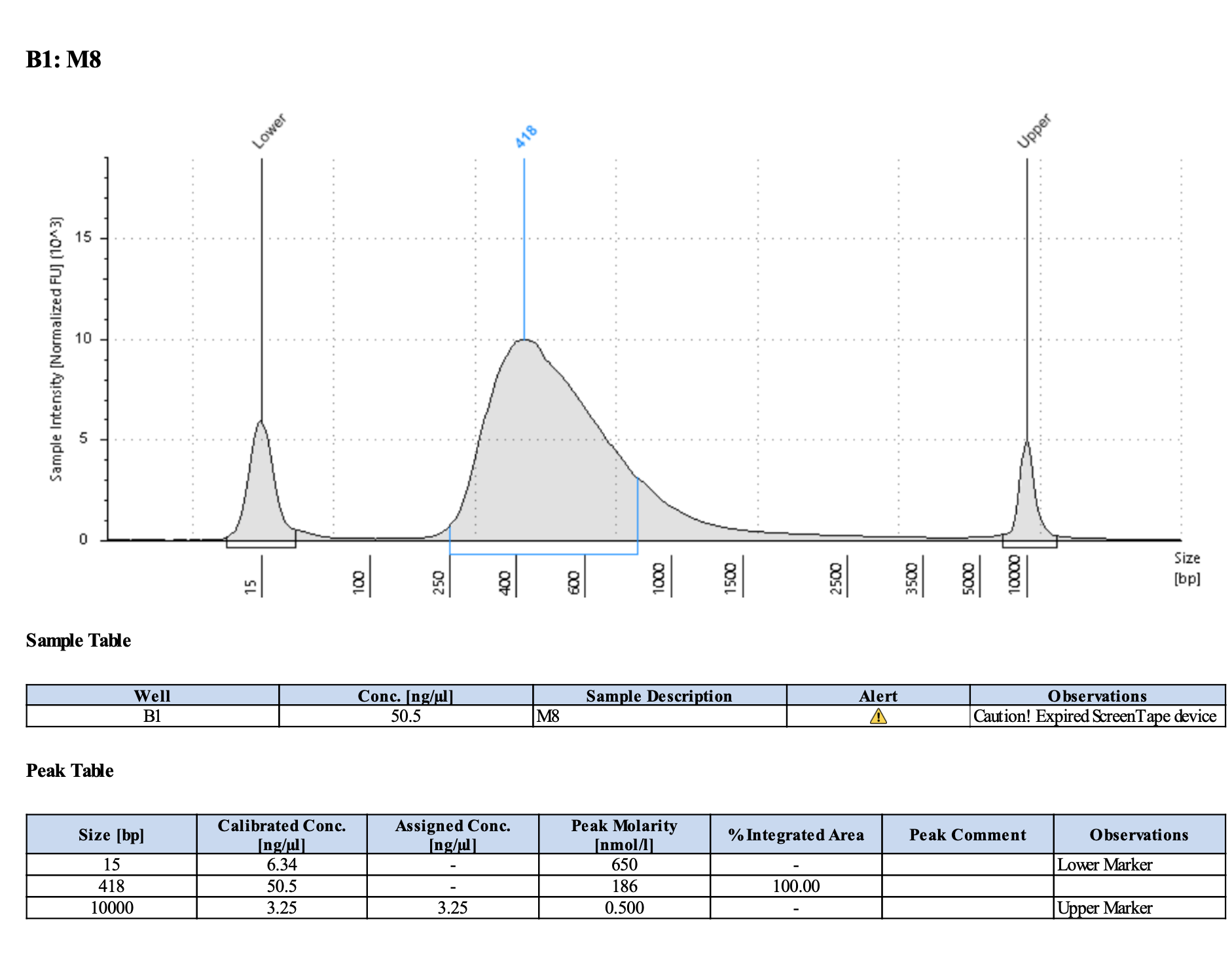
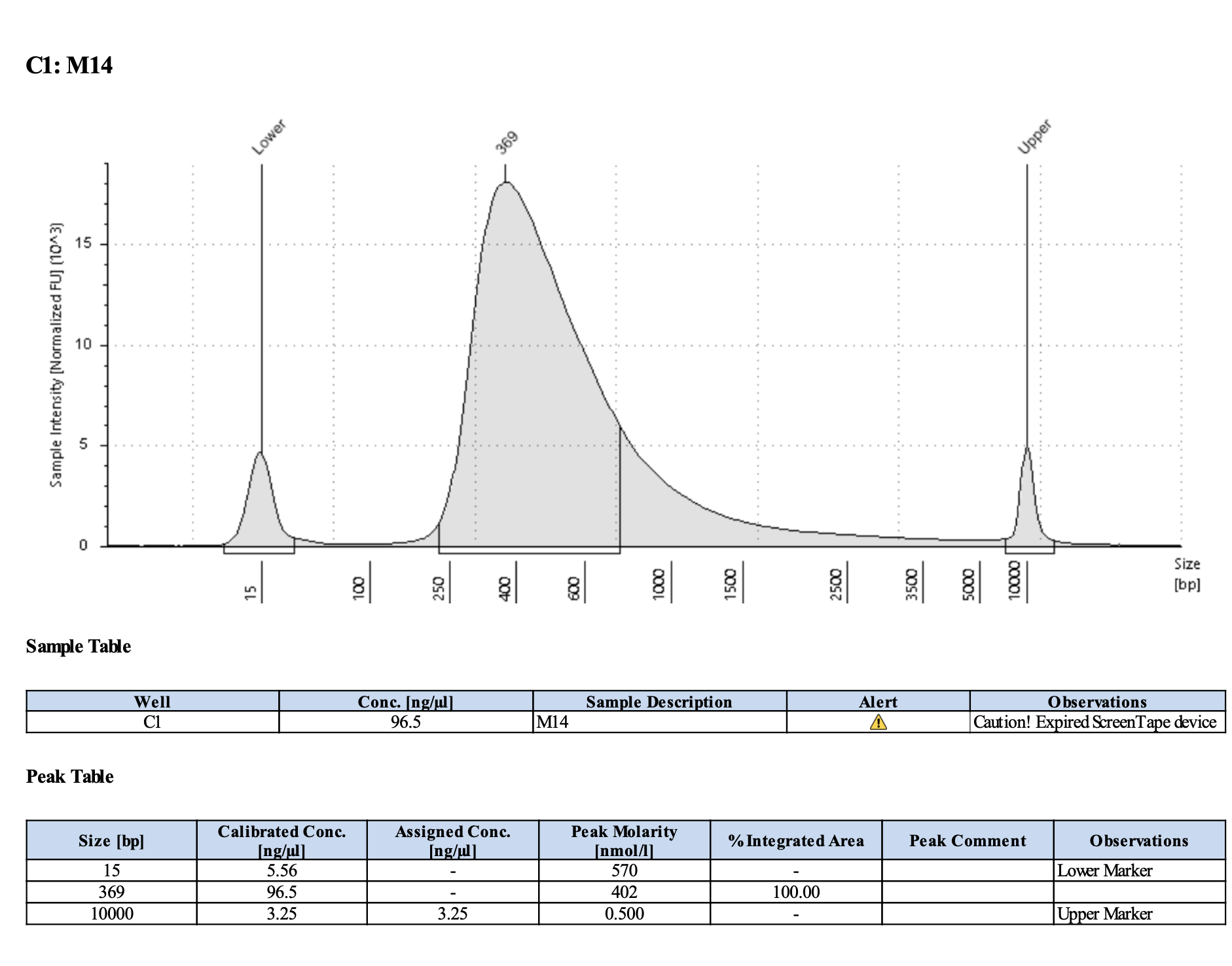
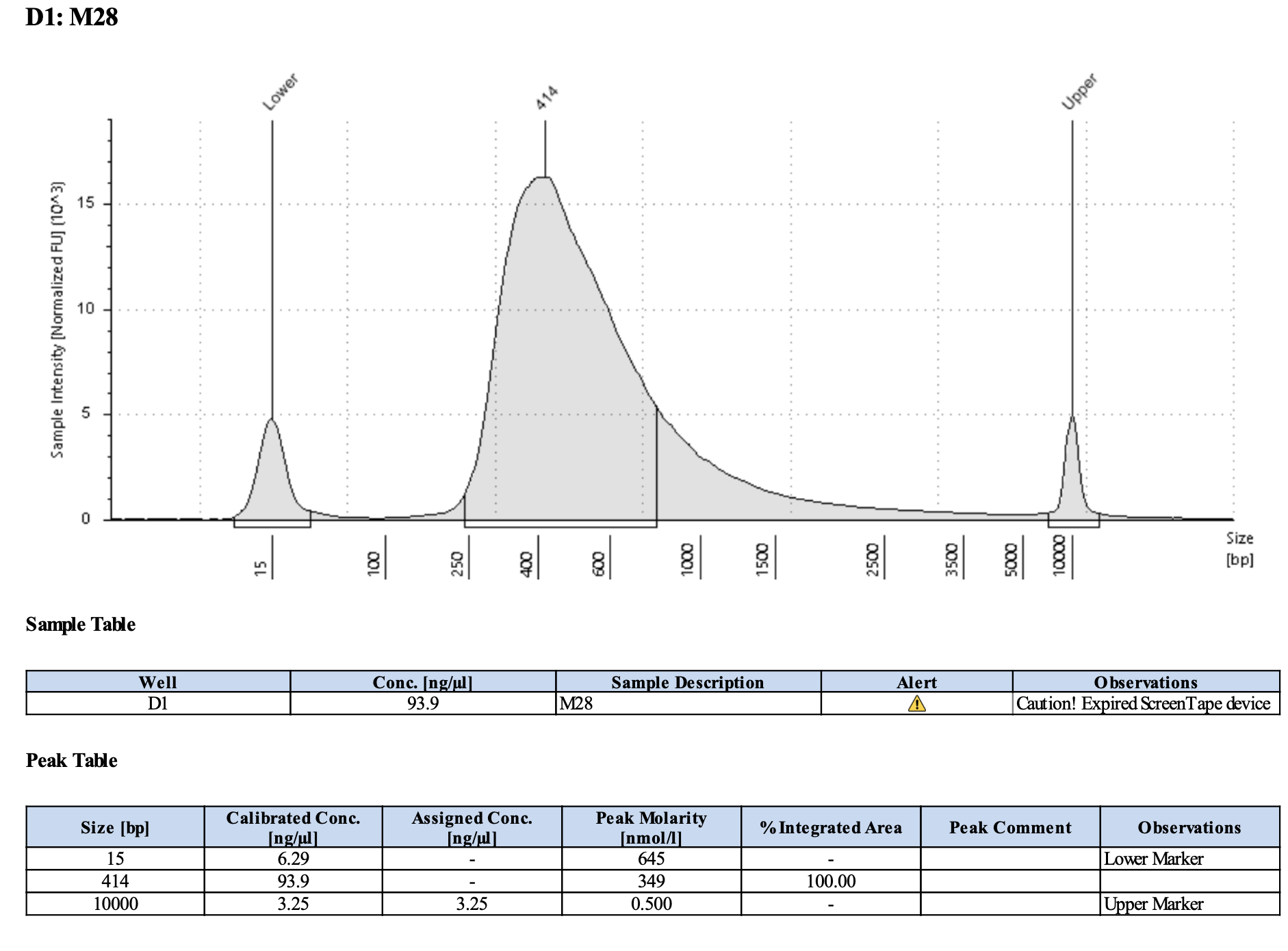
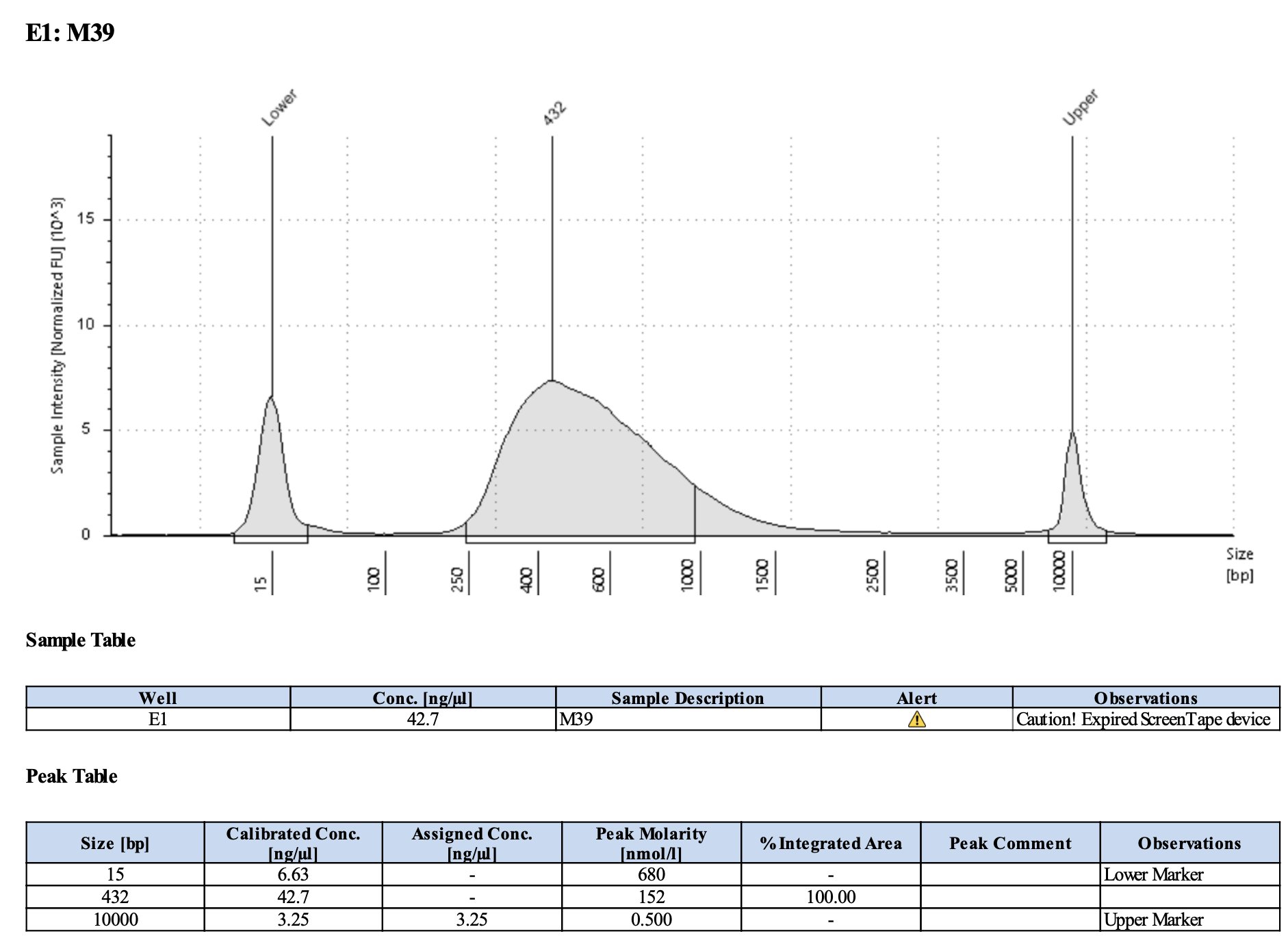
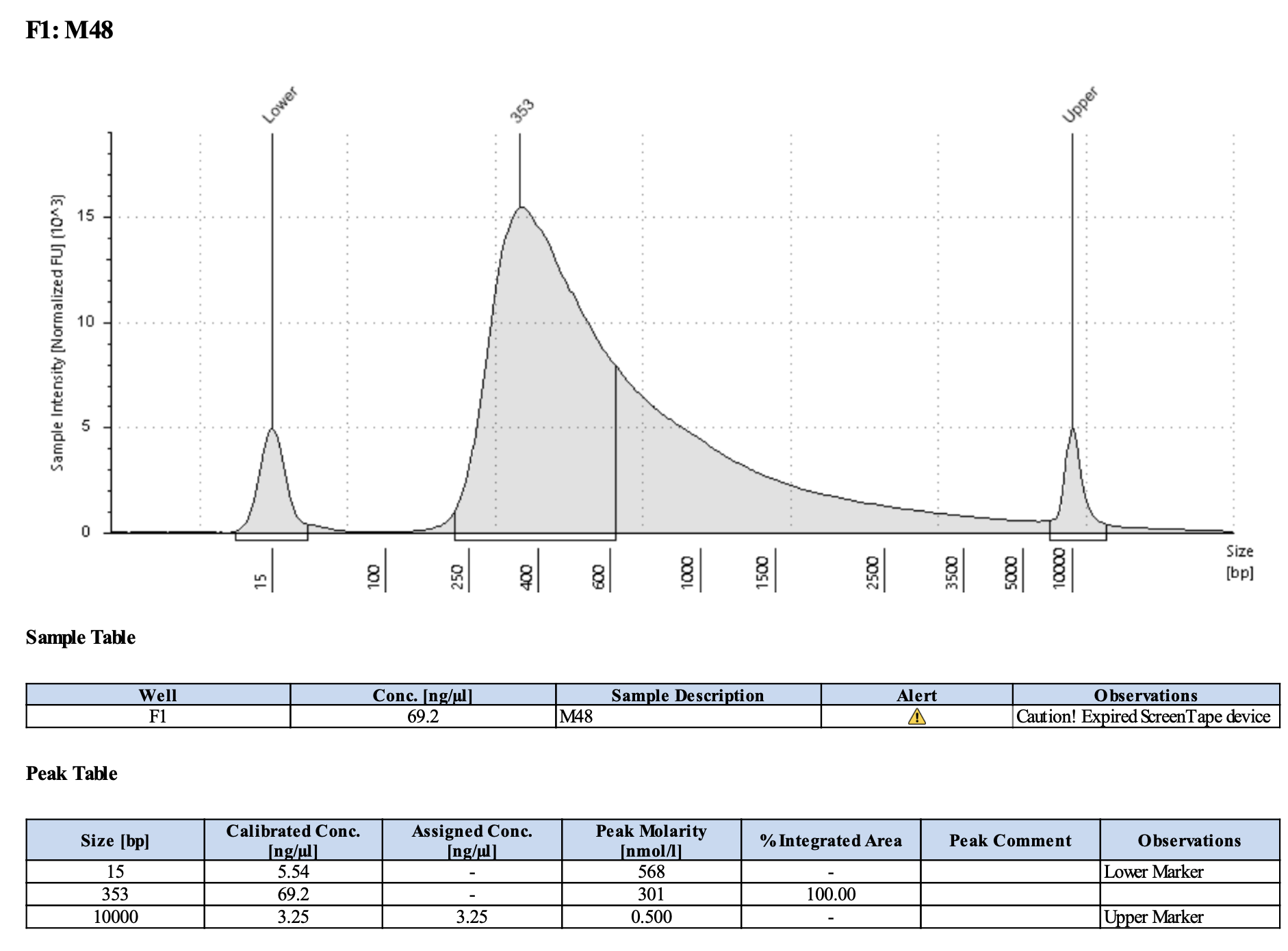
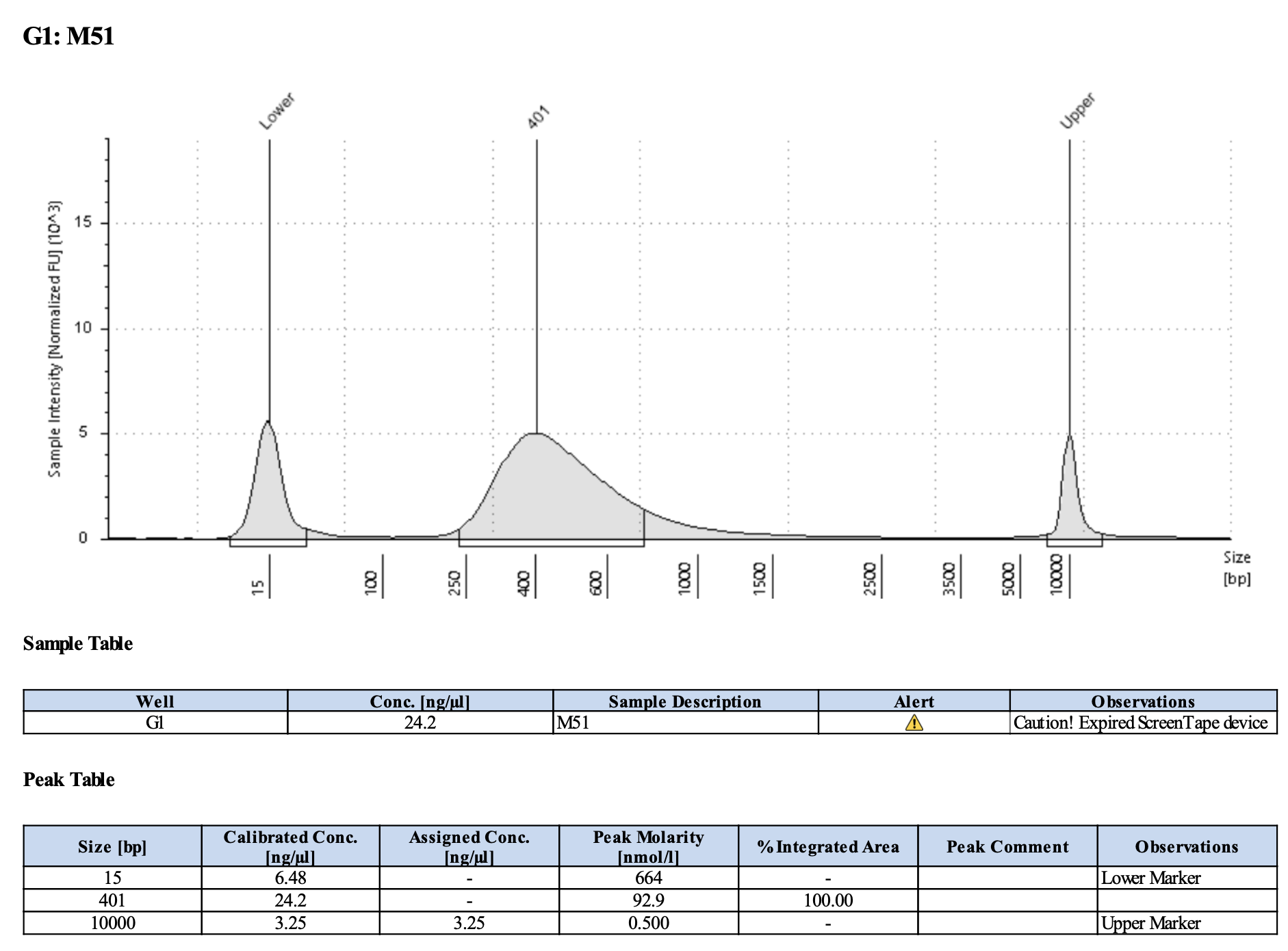
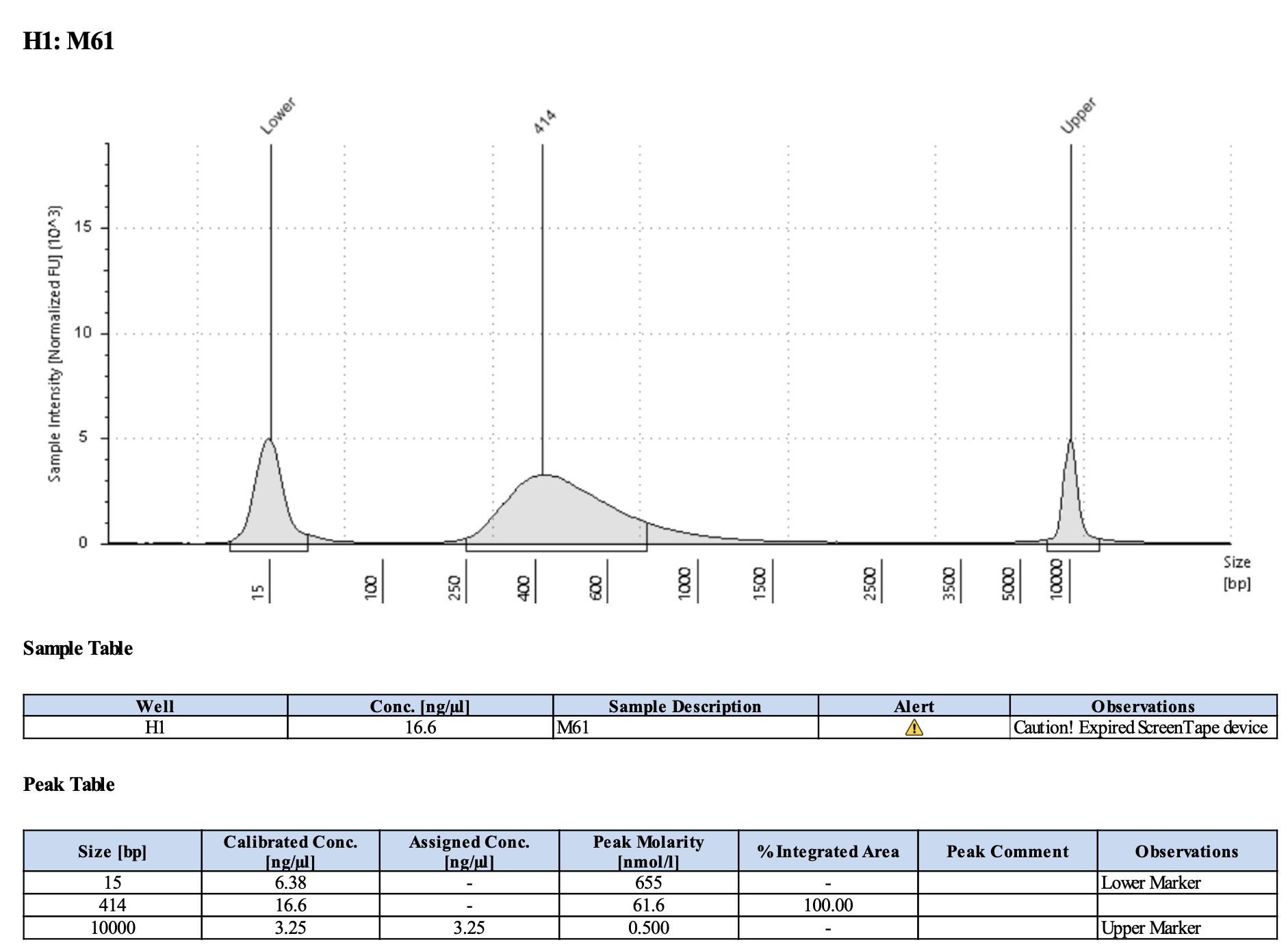
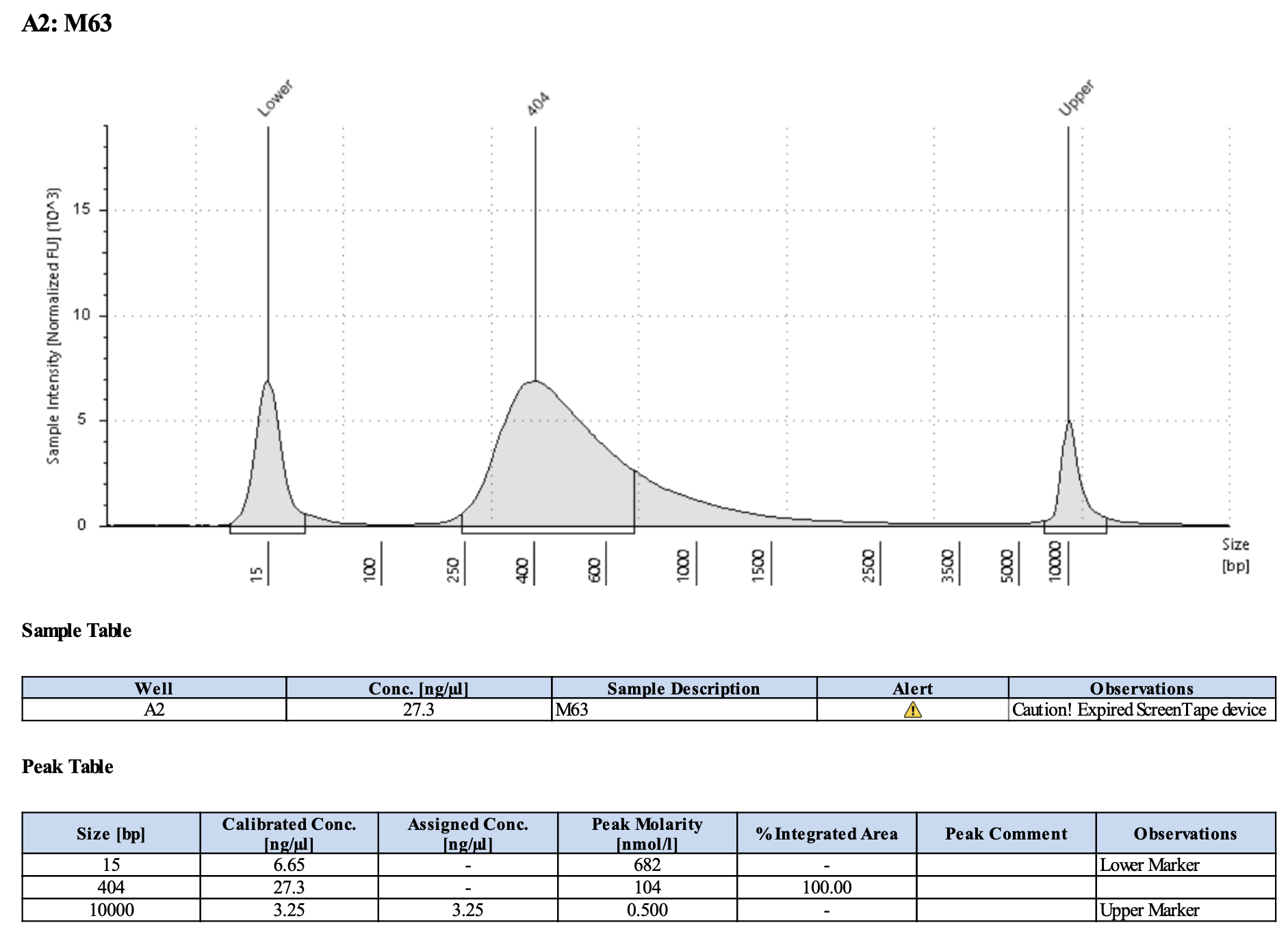
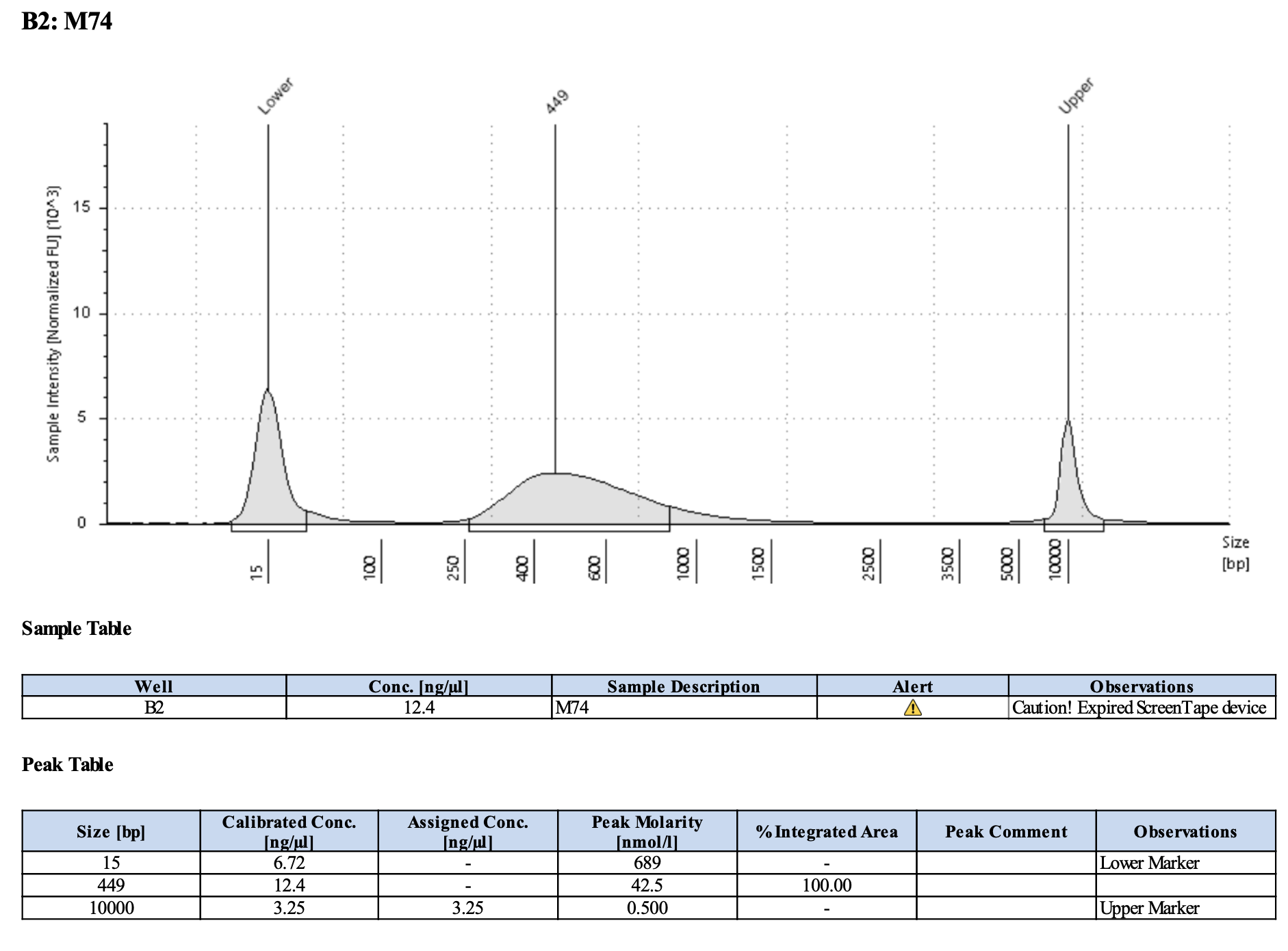
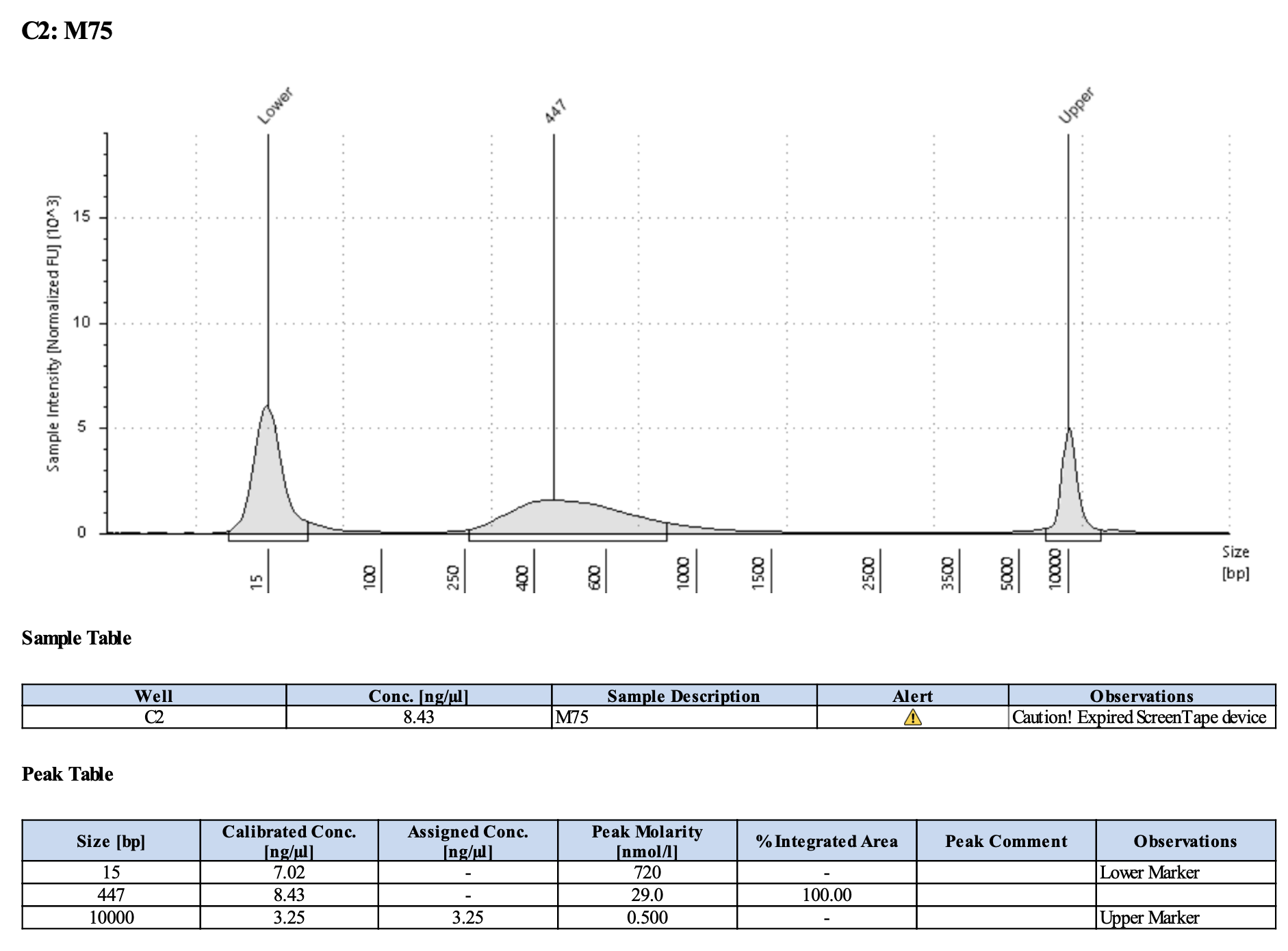
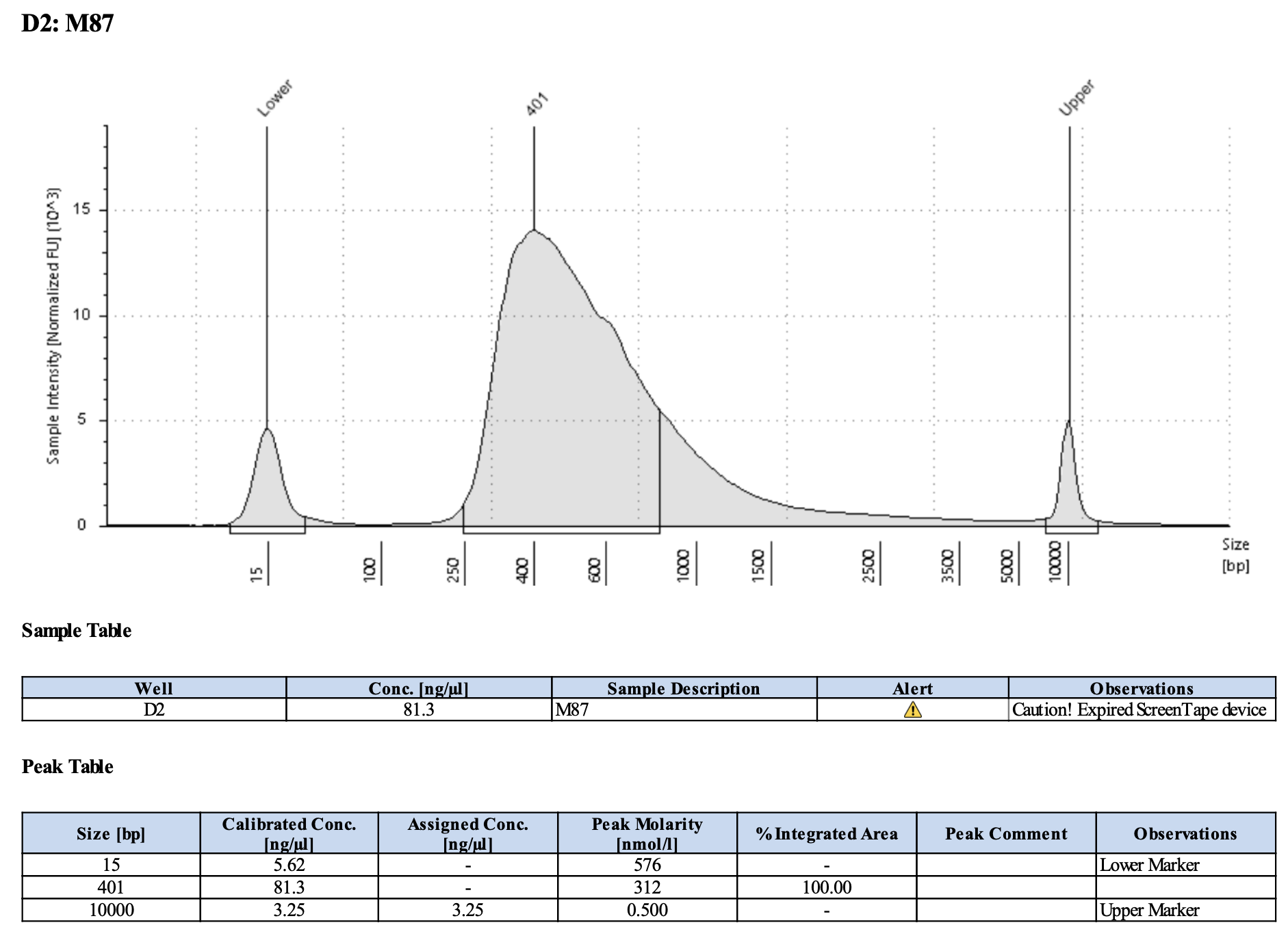
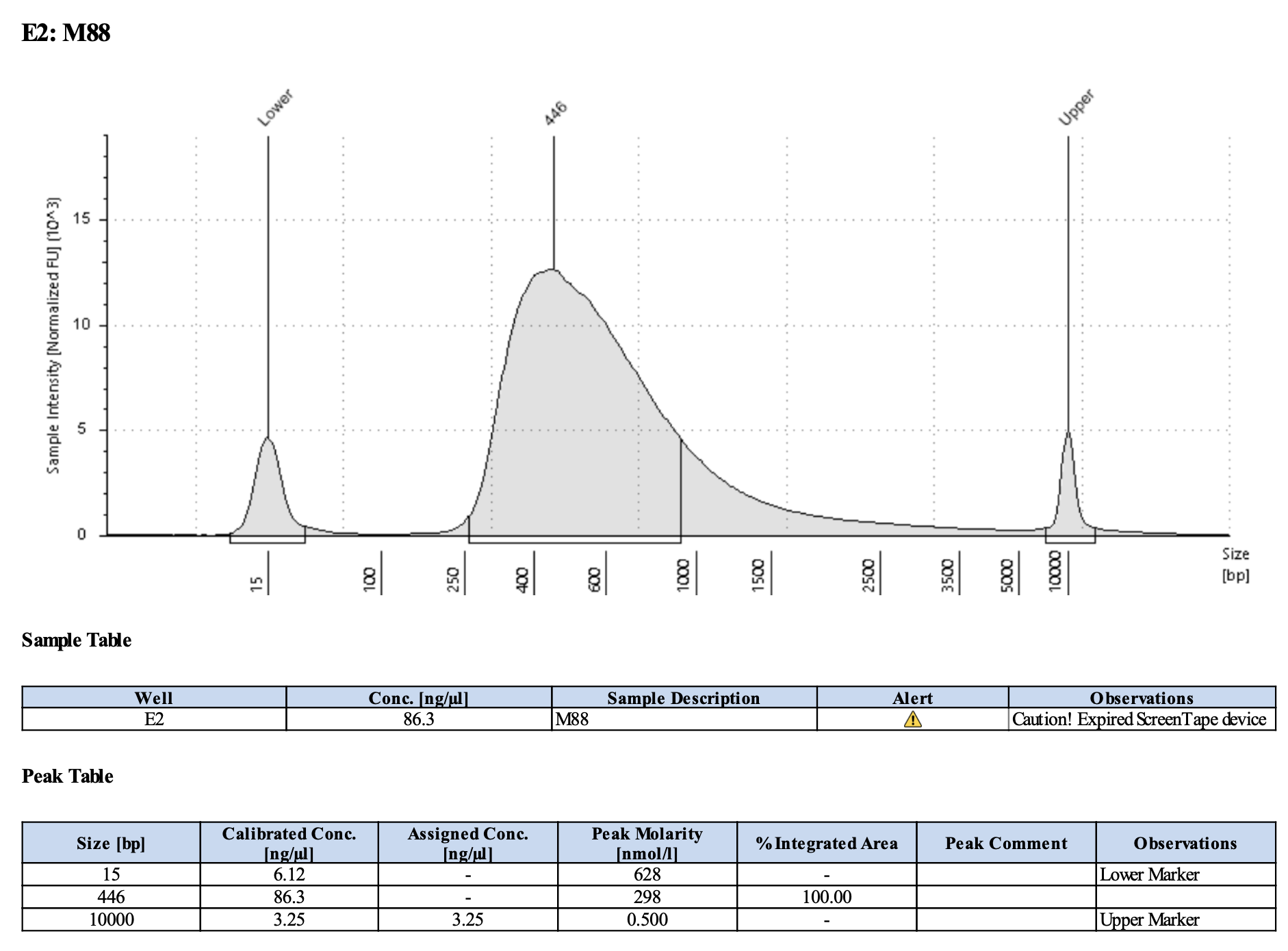
Some peaks are relatively small, but all looks good enough for sequencing.