ITS2 clean-up for Mcap C/D
ITS2 for Mcap larvae C/D Hawaii 2023 experiment
This post details information on ITS2 clean-up for the Mcap larval C/D 2023 experiment. The github for that project is linked here. ITS2 amplicon sequencing is being used to characterize the symbiont community from larval samples. See Ariana’s post about the ITS2 protocol for this project.
Samples
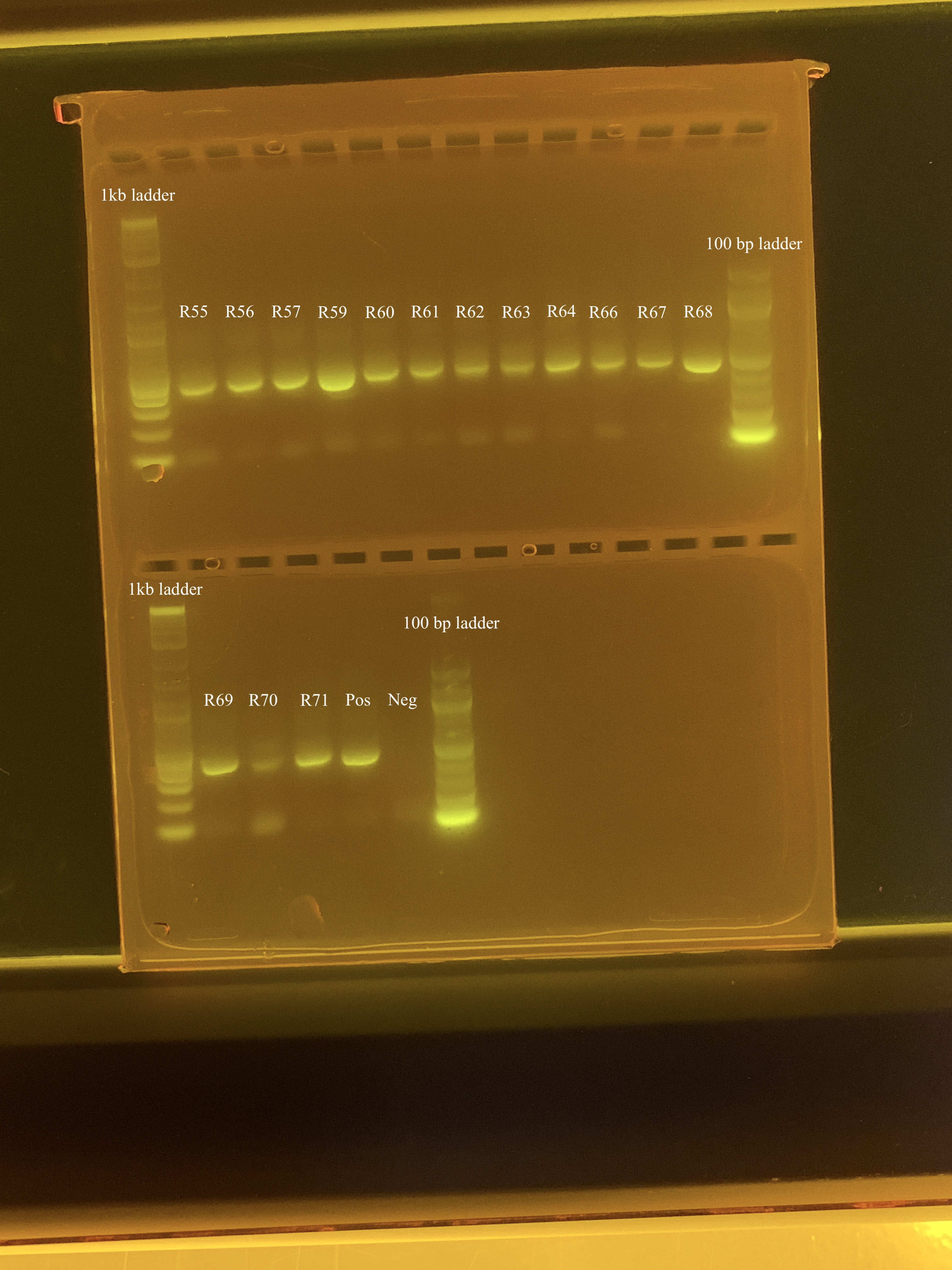
Samples (18 samples, positive control, negative control) from A. Huffmyer’s 2023 experiment were amplified but there appeared to be some contamination in the gels, as evidenced by bands ~100bp. After further troubleshooting, it was determined that these bands are likely primer dimers. Therefore, we decided to do a bead clean-up to remove the primer dimer in the samples. On 4/17/24, I ran a test clean-up on 3 samples, which adequately cleaned up the samples. Today, I cleaned the rest of the samples. Samples used today were:
- R55
- R56
- R57
- R59
- R60
- R61
- R62
- R63
- R64
- R66
- R67
- R68
- R69
- R70
- R71
- Positive control (Mcap 2020 sample)
- Negative control (ultrapure h2o)
Samples were thawed on ice. Similar to 4/17/24 clean-up, there was a weird-looking separation happening in all of the tubes (see photo in 4/17/24 post). I’m not sure what the bottom layer was, but I assumed that the PCR product was the top layer. Flicking and brief vortexing did not seem to affect it. For the protocol, I took material from the top layer.
Equipment and materials
- Kapa Pure Beads
- Tris-HCl (10 mM)
- Molecular grade ethanol
- Magnetic stand
- PCR tubes
- Gel rig + materials
Protocol
Protocol was followed according to this post. A few notes:
- After adding Kapa Beads, I incubated tubes for 15 minutes at room temperature.
- After the ethanol washes, I dried beads for ~4 minutes until beads were matte/ethanol had evaporated.
- After adding the elution buffer (10 mM Tris-HCl), I incubated tubes for 8 minutes at room temperature.
QC
Following the clean-up protocol, I ran a 2% gel for 90 minutes at 80 volts to check if the primer dimers were removed. I also used a 1kb ladder and a 100 bp ladder.
There still looks to be some faint primer dimer contamination but this is likely good enough to send for ITS2 sequencing!