Bermuda 2023 daily notebook posts
This post includes the full notebook for the July-August 2023 Diploria labyrinthiformis coral spawning and field expedition in Bermuda at the Bermuda Institute of Ocean Sciences. My project will focus on development of D. labyrinthiformis larvae through their use of energetic stores and expression of coding and non-coding transcripts. The ENCORE team (Dr. Hollie Putnam (URI), Dr. Gretchen Goodbody-Gringley (CCMI), Dr. Sam de Putron (BIOS), Dr. Yvonne Sawall (BIOS), Dr. Brett Jameson (BIOS), Dr. Chloe Carbonne (BIOS, Flo Fields (URI), and Hailey Davis (CCMI)) are also here doing a variety of experiments with D. labyrinthiformis and Porites astreoides. Githubs for the ENCORE projects are here. Github for the developmental timeseries is here.
20230730
Arrived! Flo showed me around BIOS campus and the mesocosm area. We sized the PAST spat that are here from an earlier experiment.
20230731
Mostly prep work. I unpacked the things I brought in the pelican cases and organized our supplies. I labeled tubes for my developmental time series. Because I’m hoping to link this time series to the one I did in Hawaii, I am keeping a similar labeling scheme:
ADD LABELING SCHEME
I also helped the ENCORE team with some Past work (i.e. cleaned, sized spat, made concrete for outplanting Past adults back on the reef).
The ENCORE team had a meeting today where Hollie presented TPC data from last summer.
20230801
In the morning, I did more prep work for Dlab spawning and helped with sizing the Past spat. We also sampled the Past spat and adults for DNA/RNA.
The ENCORE team had another meeting today where Brett reviewed the Past experimental design and steps moving forward. Then I presented about plans for Dlab spawning. The best paper on Dlab spawning in the Caribbean is Chamberland et al. 2017. Important to note is that these colonies were monitored in situ and the experiment took place in Curaço. They found that egg-sperm bundles were typically released 10-13 days after the full moon, with peak release at 11-12 days after the full moon:
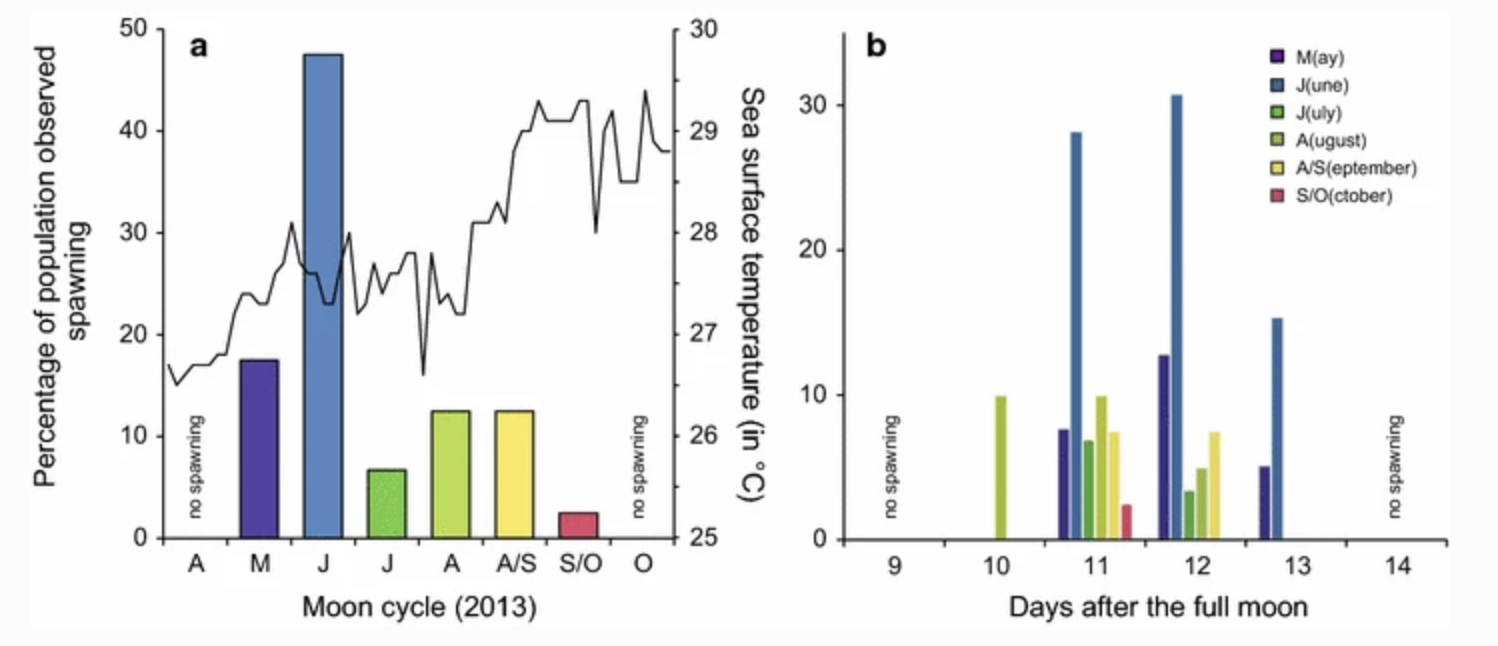
In this paper, release occurred 52 to 2 minutes before sunset. Bundles were also released in pulses. From the Chamberland et al. 2017 paper: “Typically, one section of the colony spawned for 5 ± 5 min (mean ± SD) after which all gamete release stopped, then resumed after 3–20 min. This resulted in 1–3 spawning pulses per colony per day.”
This is a figure that documents embryogenesis, larval development and settlement in the Chamberland paper.
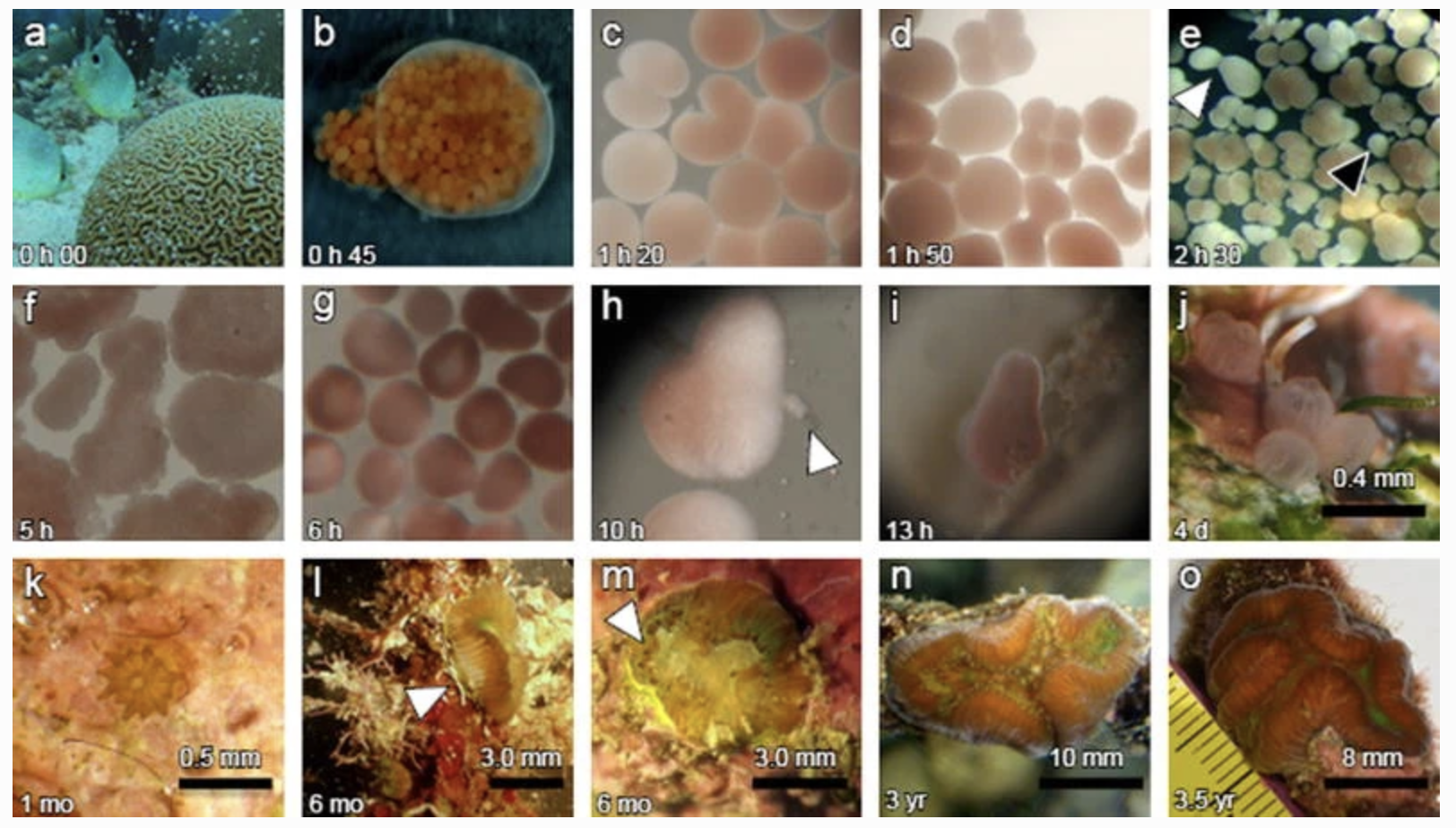
They reported that bundles typically broke apart ~45 mins after release (b). 80 mins after release, fertilized eggs undergo their first cleavage (c). 30 mins later, another cleavage occurs (d). 180 mins later, embryos break up into smaller embryos that may be viable (e). 5 hours after spawning, embryos develop into prawn chips (f). 6 hours after spawning, the onset of gastrulation begins (g). 10 hours after spawning, embryos become oval shaped (h). 13 hours after spawning, motile planulae develop (i). Larvae settled ~14 hours after CCA is provided for settlement (j). By 1 month after spawning, ~45% of recruits had acquired symbionts. I was frustrated by this paper, in that it documented development up to 13 hours post spawning and then didn’t document anything else until 4 days after spawning when they had settled. It seems that development happens rather rapidly (compared to other species like Mcap).
The paper also looked at the behavior of the individuals over time.
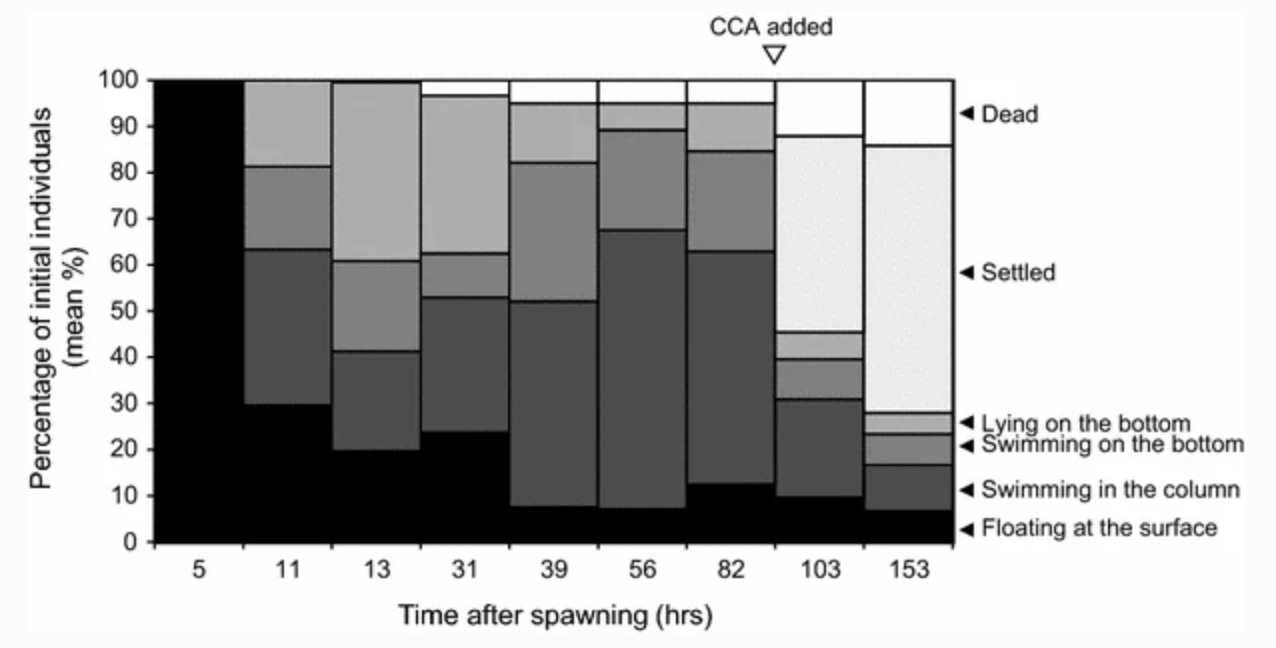
They showed that 31-39 hours after spawning, most of the individuals were either swimming in the water column, swimming on the bottom or lying on the bottom. They didn’t provide CCA until after 82 hours after spawning, but I suspect that the larvae could have settled much earlier.
After this overview, I presented my experimental overview. Essentially, it is the same experimental design as my Mcap experiment in Hawaii this year. I plan to sample at 8 developmental stages (fertilized egg, cleavage, prawn chip, early gastrula, mid gastrula, late gastrula, planula, and swimming larvae) at 2 treatments (ambient and high temperatures). n=6 replicates per treatment per time point.
The ENCORE team discussed further Dlab spawning plans. We came up with 2 tiers:
- Tier 1
- Sperm concentration calibration curve
- Eggs per bundle
- Sperm per bundle
- Egg size
- Fertilization success at ambient and high temperature
- Fertilization within a colony
- Gamete timing
- My experiment :-)
- Tier 2
- Squaricle cultures at high and ambient temperatures (n=8 replicate squaricals per treatment)
We also discussed when would be the best time to collect the adult Dlab colonies, as the weather doesn’t look good for the next few days. We decided that we will try to go on Saturday morning (8/5).
20230802
Similar to yesterday, I did prep work for Dlab in the morning. I also started writing up protocols for Dlab spawning, which will be detailed in this document in the next few days.
We had our last big ENCORE meeting today. We confirmed that we would collect 10 adult Dlab colonies on Saturday (8/5); they will be ~30 cm in diameter. Hollie (still in RI) is making the spawning booms/dresses that will be put around the coral colonies each potential spawning night. We also discussed what else we want to do with the Dlab larvae (if we get any). We decided to try and replicate the ENCORE Past experiment that recently happened over the last few weeks. For this experiment, we will expose Dlab larvae to ambient or high temperatures for the duration of their larval development. At the end of 36-48 hours, we will sample the larvae for the same metrics as they sampled
We also reviewed procedures for data storage/management, communication channels, and other housekeeping things.
20230803
Worked on protocols and made datasheets for the protocols for Dlab spawning. These will be added to this post shortly.
20230804
Cup match, no work!
20230805
We collected 10 Dlab adult coral colonies (30cm in diameter) from Bailey’s Bay Flatt this morning. The colonies are looking really happy in the mesocosms!
20230806
Lots of DLAB prep today! Hollie, Flo, Hailey and I did a lot of cleaning and organizing. Hailey and Flo moved the PAST spat to new mesocosms and set up the SMILE system in 4 of the smaller mesocosms. Hollie organized and cleaned the trailer. I prepped the different stations that we will need when the corals actually spawn. I also made 0.22 um filtered seawater today with this fancy vacuum pump (picture below). Very happy that we could find a vacuum pump or else I would have had to pump ~20 L of FSW by hand.
Hollie and I were able to get access to a spectrophotometric plate reader and do an initial run on the plate reader to see how long it might take to run the sperm calibration curve. We set it so that it would read from 280-800 nm at 10 nm increments, but it stopped before it was finished for some reason. Instead, we set it so that it would read from 280-800 nm in 20 nm increments. This worked and took ~15 minutes. Hollie may need to run it in smaller “chunks” (i.e., run from 280-400 nm at 20 nm increments, 400-600 in 20 nm increments, etc), depending on how long things are taking.
Around 1900, we started watching the corals for signs of setting. We didn’t see any by 21:00, so we called it off for the night. This is not unexpected to me, as it’s still pretty earlier after the full moon for spawning.
20230807
We have 3 temperature probes here - one on the YSI, one that I brought and one that Zoe brought. None of them read exactly the same temperature, which is unfortunate. Hollie tested them all out today and wrote on the 2 probes that we brought the offset to have the data equal to YSI if we collect temperature data using different probes. We also launched 16 Hobo UA-00x pendant loggers yesterday (labeled DLAB-1 through DLAB-16. They were zip-tied together and put in the adult tank over night to calibrate and make sure they are all reading the same temperatures.
More Dlab spawning prep! I finished labeling the sampling tubes for all the different stations for Dlab spawning. I also made 10% formalin in FSW. Hailey and Flo also finished some prep for tanks. Hollie found some good resources (Youtube video here) that details out Dlab setting and spawning. It seems like setting occurs pretty quickly (in minutes) and release follows shortly after that, so it’s unlikely that we will see setting for an hour or so prior to release.
Because of this, we decided to tent the corals preemtively in the evening. We tented the corals around 1900 and monitored until 2100. As dusk falls, the mouths of the corals start to come out, which looks like bundles in their stages of setting. After monitoring them, however, it is clear that nothing is happening with the mouths and they are not releasing bundles, as release should be pretty quick.
Below are some fun photos of tenting the Dlab colonies in their Barbie dresses!

I also started taking discrete measurements today for the adult tank and Basins 1-4. I won’t start measuring flow rates until we are using the squaricals. The data for today is in the table below:
| date | time | tank | treatment | tris.date | temp.C | sal.psu | pH.mV | pH.nbs | par | flow.mL.5s | initials | notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8/7/23 | 10:00 | Adult | Ambient | 20230711 | 27.8 | 36.09 | -58.6 | 8.02 | 120 | NA | JA | Temperature taken w/ YSI |
| 8/7/23 | 10:00 | Basin 2 | High | 20230711 | 30.3 | 36.08 | -59.5 | 8.02 | 203 | NA | JA | Temperature taken w/ YSI |
| 8/7/23 | 10:00 | Basin 1 | Ambient | 20230711 | 27.8 | 36.08 | -60.3 | 8.03 | 230 | NA | JA | Temperature taken w/ YSI |
| 8/7/23 | 10:00 | Basin 4 | High | 20230711 | 30.6 | 36.09 | -59.6 | 8.02 | 114 | NA | JA | Temperature taken w/ YSI |
| 8/7/23 | 10:00 | Basin 3 | Ambient | 20230711 | 28 | 36.1 | -60.6 | 8.05 | 131 | NA | JA | Temperature taken w/ YSI |
| 8/7/23 | 13:30 | Adult | Ambient | 20230711 | NA | NA | NA | NA | 462 | NA | JA | Temperature taken w/ YSI |
| 8/7/23 | 13:30 | Basin 1 | Ambient | 20230711 | 27.7 | 36.12 | -59.5 | 8.03 | 698 | NA | JA | Temperature taken w/ YSI |
| 8/7/23 | 13:30 | Basin 2 | High | 20230711 | 30.4 | 36.13 | -58.4 | 8.03 | 665 | NA | JA | Temperature taken w/ YSI |
| 8/7/23 | 13:30 | Basin 3 | Ambient | 20230711 | 27.5 | 36.11 | -60.4 | 8.05 | 451 | NA | JA | Temperature taken w/ YSI |
| 8/7/23 | 13:30 | Basin 4 | High | 20230711 | 30.5 | 36.12 | -61.3 | 8.05 | 368 | NA | JA | Temperature taken w/ YSI |
| 8/7/23 | 19:20 | Adult | Ambient | 20230711 | 29 | 36.19 | -62.8 | 8.11 | 30 | NA | JA | Temperature taken w/ YSI |
| 8/7/23 | 19:20 | Basin 1 | Ambient | 20230711 | 27.8 | 36.2 | -63.9 | 8.08 | 19 | NA | JA | Temperature taken w/ YSI |
| 8/7/23 | 19:20 | Basin 2 | High | 20230711 | 30.3 | 36.23 | -60.2 | 8.06 | 14 | NA | JA | Temperature taken w/ YSI |
| 8/7/23 | 19:20 | Basin 3 | Ambient | 20230711 | 27.8 | 36.19 | -62.5 | 8.07 | 13 | NA | JA | Temperature taken w/ YSI |
| 8/7/23 | 19:20 | Basin 4 | High | 20230711 | 30.3 | 36.19 | -63.6 | 8.07 | 9 | NA | JA | Temperature taken w/ YSI |
20230808
I have finialized the Dlab spawning and experimental protocols for the most part. We have also assigned who will be doing which protocols.
Monitoring protocol - ALL
- DLAB colonies will be collected on Saturday (8/5). Put colonies in the large mesocosm tank with 2 hobo loggers. Monitor temperature, salinity, pH and light 2-3 times per day in tanks.
- Once colonies are in the tanks, monitor for spawning.
- At 7:30pm - 9:30pm every night, put spawning booms/dresses over each colony to collect egg-sperm bundles once they are released.
Spawning protocol - ALL
- Once the corals begin to spawn, collect egg-sperm bundles using a 50 mL falcon tube with a stop cock. The bundles will be positively buoyant and float to the surface. Open the stop cock to let out some of the water so the bundles are more concentrated.
- Pour the bundles into a mesh filter sitting in a bowl. Add FSW if the bundles are not submerged enough.
- Add bundles from all colonies to the bowl. Swirl the bowl around gently to facilitate the breaking up of the bundles.
- Once bundles are broken up, move the mesh filter with the eggs into a bowl with fresh FSW and swirl the filter gently to rinse any remaining sperm from the eggs. Repeat this in two other bowls to fully rinse the sperm from the eggs.
- The first bowl (where eggs and sperm were separated) now has concentrated sperm in FSW. The mesh filter now has concentrated eggs in FSW. Use these pools for subsequent experiments.
Sperm calibration curve - HOLLIE
- From the bowl of concentrated sperm, obtain the most concentrated sperm possible in Tube A.
- Create a serial dilution by moving 500 uL of well-mixed sperm to a new 50 mL tube (Tube B) and adding 49.5 mL of FSW.
- Repeat this dilution for Tubes C, D, E, and F. Tube G should only be FSW.
- Write the time which sperm was added on each tube.
- Measure absorbance of each tube in a 96-well plate at 280-800 nm at 20 nm steps.
- Invert the 50 mL tubes 5x before loading 200µl samples into the plate.
- Add 200 uL of sperm to each well in duplicate and run immediately
- Move 400 uL of sperm from each tube into a 1.5 mL tube. Add 100 uL of 10% formalin to each tube. Store tubes at 4°C.
- Stain the sperm with trypan blue
- Count the sperm in each tube 6x on a haemocytometer. The counts can be done later on the fixed sample.
- Amount of pooled sperm needed: 50 mL
- Amount of pooled eggs needed: 0
Sperm Concentration Test - HOLLIE
Following Oliver & Babcock 1992
- From Tubes A-G, move 9 mL of liquid into 20 mL scintillation vials
- 5 replicate vials per concentration
- Add 20 eggs from the pool into each vial.
- Submerge vials in ambient water for 60 minutes.
- After 60 minutes, remove each vial, pour contents through a 100 um cell strainer, move contents to a 1.5 mL tube and add 100 uL of 10% formalin. Store tubes at 4°C.
- Amount of pooled sperm needed: 0
- Amount of pooled eggs needed: 35 vials x 20 eggs = 700 eggs total
Eggs per bundle, sperm per bundle & egg size - BRETT
- Place 1 egg sperm bundle from a colony into a well in a 96-well plate. Remove as much water from each well as possible.
- 12 replicate bundles per colony
- Add 100 uL of FSW to each well.
- Allow the bundle to break apart.
- Add 50 uL of 10% formalin to each well.
- Put the cover on the 96-well plate and wrap plate securely in parafilm.
- Store plates at 4°C.
- When ready to analyze, remove the sperm in each well into a 1.5 mL tube.
- Stain the sperm with trypan blue
- Count the sperm in each tube 6x on a haemocytometer. The counts can be done later on the fixed sample.
- Resuspend the eggs in 50 uL of 10% formalin.
- When ready to analyze, move eggs onto a petri dish and image them under a dissecting scope with a size standard for counting and size analysis. Use ImageJ to extract egg diameter and planar area.
- Amount of pooled sperm needed: 0
- Amount of pooled eggs needed: 0
Fertilization at ambient and high temperatures with bundle bundle crosses - HAILEY
- Add 9 mL of concentrated sperm to 20 mL scintillation vials.
- From the pool, add 10 eggs to each vial.
- Allocate vials into ambient and high treatments (n=30 vials per treatment).
- After XXXX (60 mins?), remove 10 vials per treatment. Pour contents through a 100 um cell strainer, move contents to a 1.5 mL tube and add 100 uL of 10% formalin. Store tubes at 4°C.
- Repeat step 4 after XXXX (90 mins?) and XXXX (120 mins?).
- Amount of pooled sperm needed: 9 mL x 60 vials = 540 mL
- Amount of pooled eggs needed: 10 eggs x 60 vials = 600 eggs
Not sure how long we should let fertilization to occur for…the Chamberland paper said they allowed to fertilize for 90 mins, but the youtube video said 30-45 mins. Need to talk to Hollie about changing these numbers.
Fertilization in same colony - HAILEY
- From one colony, use a plastic transfer pipette and move 10 egg-sperm bundles into 20 mL scintillation vials. Add 9 mL of FSW to each vial.
- n=5 replicate vials per colony
- Submerge vials in ambient temperature for XXX (60 mins?). Gently rock vials every 5-10 minutes to facilitate the breaking up of bundles.
- After XXXX (60 mins?), remove 10 vials per treatment. Pour contents through a 100 um cell strainer, move contents to a 1.5 mL tube and add 100 uL of 10% formalin. Store tubes at 4°C.
- Amount of pooled sperm needed: 0
- Amount of pooled eggs needed: 0
Timing of egg and sperm viability - FLO
Following Oliver & Babcock 1992
- Add 9 mL of concentrated sperm to 50 20 mL scintillation vials.
- Every 30 minutes after spawning, add 10-20 eggs from the pool into each vial.
- 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5 hours after spawning (10 time points total)
- 5 replicate vials per time point
- After XXX (60 mins?), pour the contents of each vial through a 100 um cell strainer, move contents to a 1.5 mL tube and add 200 uL of 10% formalin. Store tubes at 4°C.
- Amount of pooled sperm needed: 9 mL x 50 vials = 450 mL
- Amount of pooled eggs needed: 10 eggs x 50 vials = 500 eggs
Pooled fertilization - ALL
- Once the eggs and sperm have been taken for the experiments above, combine the eggs and sperm into a bowl and/or falcon tubes for fertilization.
- Allow fertilization to occur for 60 mins?
- After fertilization, rinse the eggs 3x to wash away any residual sperm.
Jill’s developmental time series - JILL
- Once fertilization is finished in the pooled group and eggs have been rinsed, take an aliquot of 5-10 uL of concentrated eggs and count under a dissecting scope. Calculate the number of eggs in 200 uL.
- Add 100-200 uL of concentrated eggs to 50 mL falcon tubes with 40 mL of FSW.
- Put half of the 50 mL falcon tubes in incubators set to ambient and high temperatures. Add 2 loggers per incubator.
- Rock tubes every 1 hour to prevent build up and stagnant water.
- At several developmental time points, sample from n=6 falcon tubes per treatment.
- Pour the contents of the tube through a 100 um cell strainer.
- Put cell strainer in a 6 well plate so the bottom is submerged, but the embryos are still concentrated.
- Take a 20 uL subsample of concentrated embryos and preserve them in formalin (S tubes).
- Move half of the concentrated embryos into a 1.5 mL tube and preserve with 500 uL of DNA/RNA shield (M tubes).
- Move the other half of concentrated embryos into a 1.5 mL tube and snap-freeze in LN2 (P tubes)
- TIME POINTS (these may change depending on how development appears to be progressing): 30 mins pf, 1.5 hpf, 5 hpf, 6 hpf, 8 hpf, 10 hpf, 13 hpf, 24 hpf, 36 hpf?
ENCORE experiments - ALL
- Once fertilization is finished in the pooled group and eggs have been rinsed, pour XXX amount of eggs into each squarical of the ambient and high treatments (n=8 squaricals per treatment).
- Monitor squaricals every hour.
- If embryos look like they are clumping along the edges of the squaricals, use a plastic pipette to gently stir them.
- If the surface of the water looks dirty, gently run a Kim wipe over the surface of the water to remove debris.
- If the embryos appear to be dying in large numbers, drain the squarical through a 100 um sieve and rinse the remaining embryos with FSW. Refill the squarical and put the remaining embryos back inside.
- Take discrete measurements every six hours (temperature, pH, salinity, flow).
- After 36 hours, drain the squarical as detailed above (2c) and concentrate the larvae for sampling and settlement counts.
Count out 100 larvae into a 1.5 mL tube and snap freeze them for physiology.
- n=1 replicate per squarical?
- Count out 100 larvae into a 1.5 mL tube and preserve them with 700 uL of DNA/RNA shield for molecular analysis.
- n=1 replicate per squarical?
- Count out 30 larvae into a 1.5 mL tube and preserve them with 200 uL of 10% formalin for size analysis.
- n=1 replicate per squarical?
- Set aside larvae for respiration runs (Jill will run respiration and potentially TPCs).
- Set aside larvae for settlement in settlement chambers.
Yay protocols! There are still some timing things and replicate numbers I need to work out, but that’s okay. We tented and watched the corals from 18:45-20:45 tonight, but no signs of spawning. Still pretty early after the full moon. While we were watching last night, we talked through protocols to make sure everyone was on the same page. I also filled up my 50 mL falcon tubes (100 in total) with FSW and loaded them into the incubators so they will be at temperature when the babies arrive.
Here are the daily measurements from today:
| date | time | tank | treatment | tris.date | temp.C | sal.psu | pH.mV | pH.nbs | par | flow.mL.5s | initials | notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8/8/23 | 10:20 | Adult | Ambient | 20230711 | 27.3 | 36.25 | -59.7 | 8.04 | 238 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 10:20 | Basin 1 | Ambient | 20230711 | 27.9 | 36.28 | -60.7 | 8.05 | 409 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 10:20 | Basin 2 | High | 20230711 | 30.3 | 36.28 | -60.8 | 8.04 | 447 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 10:20 | Basin 3 | Ambient | 20230711 | 27.6 | 36.27 | -61.8 | 8.07 | 346 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 10:20 | Basin 4 | High | 20230711 | 30.3 | 36.27 | -61.2 | 8.05 | 278 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 10:20 | Adult | Ambient | 20230711 | 27.5 | 36.3 | -62 | 8.07 | 382 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 10:20 | Basin 1 | Ambient | 20230711 | 27.8 | 36.26 | -61.2 | 8.05 | 691 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 10:20 | Basin 2 | High | 20230711 | 30.3 | 36.28 | -60.9 | 8.04 | 729 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 10:20 | Basin 3 | Ambient | 20230711 | 28.2 | 36.29 | -61 | 8.05 | 481 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 10:20 | Basin 4 | High | 20230711 | 30.3 | 36.33 | -59 | 8.01 | 423 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 13:30 | Adult | Ambient | 20230711 | 27.5 | 36.3 | -62 | 8.07 | 382 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 13:30 | Basin 1 | Ambient | 20230711 | 27.8 | 36.26 | -61.2 | 8.05 | 691 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 13:30 | Basin 2 | High | 20230711 | 30.3 | 36.28 | -60.9 | 8.04 | 669 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 13:30 | Basin 3 | Ambient | 20230711 | 28.2 | 36.29 | -61 | 8.05 | 481 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 13:30 | Basin 4 | High | 20230711 | 30.3 | 36.33 | -59 | 8.01 | 423 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 18:45 | Adult | Ambient | 20230711 | 27.7 | 35.27 | -62.8 | 8.09 | 132 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 18:45 | Basin 1 | Ambient | 20230711 | 27.9 | 36.28 | -63.1 | 8.09 | 111 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 18:45 | Basin 2 | High | 20230711 | 30.3 | 36.32 | -60.5 | 8.04 | 73 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 18:45 | Basin 3 | Ambient | 20230711 | 27.5 | 36.29 | -60.2 | 8.03 | 47 | NA | JA | Temperature taken w/ YSI |
| 8/8/23 | 18:45 | Basin 4 | High | 20230711 | 30.3 | 36.3 | -58.2 | 8.02 | 26 | NA | JA | Temperature taken w/ YSI |
The temperatures are pretty consistent in the ambient and high tanks. The light is variable between tanks because of the shade each one is receiving and the angle of the sun whenever I am taking measurements.
This afternoon, I also downloaded all the Hobo logger data from the calibration and temperatures look consistent across loggers. I redeployed them so that there are now loggers in the incubators (1 logger per incubator, 2 total), 2 loggers in the adult tanks, and 3 loggers per basin (4 basins x 3 loggers per basin = 12 loggers total).
20230809
Updated Dlab protocols and lipid assay protocol.
I went through papers and posts on the Coral Spawning Research group on Facebook today to investigate Dlab spawning times. I compiled the following information:
| Location | Year | Month(s) | DAFM* | Time of spawning | In situ v. ex situ | Notes | Source |
|---|---|---|---|---|---|---|---|
| Bermuda | 1986 | July | Unknown | Unknown | In situ | Wyers et al. 1991 | |
| Curacao | 2013 | May-October | 10 to 13 | 52 to 2 mins before sunset | In situ | Peak in June, 11-12 days after full moon | Chamberland et al. 2017 |
| Colombian Caribbean | 1997-98 | May/June | Unknown | Unknown | NA | Based on histological samples, spawning not observed | Alvarado et al. 2004 |
| Puerto Rico | 2001-02 | April-May | 7 to 10 | Spawned after 11pm | In situ | Weil & Vargas 2009 | |
| Mexican Caribbean | 2017-18 | July-September | 10 to 11 | Monitored 1 hour before sunset | In situ | Grosso-Becerra et al. 2021 | |
| Upper Florida Keys | 2022 | April | 10 | 18:20 | In situ | NOAA 2022 report | |
| Bonaire | 2011 | May-June | 10 to 11 | 18:15 to 19:01 | In situ | Muller & Vermeij 2011 | |
| Dominican Republic | 2020 | June | 11 | 75 to 50 minutes before sunset | Ex situ | Garcia et al. 2022 | |
| Dominican Republic | 2019-20 | May-June | 10 to 11 | Ranged from 1:40 minutes before sunset to 1:15 mins after sunset | Mix | Sellares-Blasco et al. 2021 | |
| Curacao | 2012 | May | Unknown | Just before nightfall | In situ | Quere & Nugues 2015 | |
| Greater Antilles | 2023 | May-June | Unknown | 18:04 to 18:26 | In situ | Coral spawning research FB page | Manuel Alejandro Rodriguez Redondo |
| Florida | 2023 | May | Unknown | 16:30 to 16:45 | Ex situ | Coral spawning research FB page | Liv Williamson |
| Dominica | 2023 | July | Unknown | 17:31 to 17:45 | Unknown | Coral spawning research FB page | Simon Walsh |
| Florida | 2023 | May | 13 | 16:58 to 17:26 | Ex situ | Coral spawning research FB page | Rachel Serafin Morgan |
| Florida | 2023 | May | 10 | 18:30 | In situ | Coral spawning research FB page | Amanda Bourque |
| Bonaire | 2020 | June | 11 to 12 | 18:10 to 18:50 | In situ | Coral spawning research FB page | Francesca Virdis; Reef Renewal |
| Dominican Republic | 2020 | May | 10 to 11 | 17:57 to 18:25 | In situ | Coral spawning research FB page | Maria Villapando; FUNDEMAR |
| Puerto Rico | 2019 | August | Unknown | 17:47 to 18:50 | In situ | Coral spawning research FB page | Alfredo MA |
| Dominica | 2023 | June | 11 | 17:30 | In situ | Coral spawning research FB page | Marine Aimar |
| Florida | 2023 | May | 13 | 18:54 to 19:40 | Ex situ | Coral spawning research FB page | Midori Mendoza |
| Florida | 2023 | May | Unknown | 18:40 to 18:55 | In situ | Coral spawning research FB page | Liv Williamson |
| Florida | 2021 | May | Unknown | 17:21 to 17:26 | Ex situ | Coral spawning research FB page | Rachel Serafin Morgan |
| Florida | 2023 | June | 10 | 18:12 to 18:45 | Ex situ | Coral spawning research FB page | Jenny Lee |
*DAFM = Days After Full Moon
I still need to look on Youtube to see if there are videos relating to Dlab spawning times.
We tented the corals around 18:45 and watched until 2100. No spawning tonight! When we turned the pump and chiller back on for mesocosm 14, the water stopped running to all tanks. We tried turning the chillers and the pumps back on, but it didn’t work. We contacted Kevin about the water issue, but the corals should be fine for the night. The pumps are still recirculating the water in the mesocosms.
Here are the daily measurements from today:
| date | time | tank | treatment | tris.date | temp.C | sal.psu | pH.mV | pH.nbs | par | flow.mL.5s | initials | notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8/9/23 | 8:50 | Adult | Ambient | 20230711 | 27.5 | 36.34 | -60.7 | 8.05 | 73 | NA | JA | Temperature taken w/ YSI |
| 8/9/23 | 8:50 | Basin 1 | Ambient | 20230711 | 27.7 | 36.33 | -61.9 | 8.08 | 183 | NA | JA | Temperature taken w/ YSI |
| 8/9/23 | 8:50 | Basin 2 | High | 20230711 | 30.6 | 36.32 | -61.9 | 8.06 | 111 | NA | JA | Temperature taken w/ YSI |
| 8/9/23 | 8:50 | Basin 3 | Ambient | 20230711 | 27.5 | 36.34 | -62 | 8.07 | 94 | NA | JA | Temperature taken w/ YSI |
| 8/9/23 | 8:50 | Basin 4 | High | 20230711 | 30.3 | 36.34 | -60.2 | 8.03 | 151 | NA | JA | Temperature taken w/ YSI |
| 8/9/23 | 13:30 | Adult | Ambient | 20230711 | 29 | 36.3 | -62.8 | 8.08 | 464 | NA | JA | Temperature taken w/ YSI |
| 8/9/23 | 13:30 | Basin 1 | Ambient | 20230711 | 27.9 | 36.33 | -61.4 | 8.06 | 656 | NA | JA | Temperature taken w/ YSI |
| 8/9/23 | 13:30 | Basin 2 | High | 20230711 | 31 | 36.34 | -58.8 | 8.01 | 742 | NA | JA | Temperature taken w/ YSI |
| 8/9/23 | 13:30 | Basin 3 | Ambient | 20230711 | 27.6 | 36.29 | -62.2 | 8.08 | 526 | NA | JA | Temperature taken w/ YSI |
| 8/9/23 | 13:30 | Basin 4 | High | 20230711 | 30.5 | 36.32 | -62.2 | 8.06 | 328 | NA | JA | Temperature taken w/ YSI |
| 8/9/23 | 18:30 | Adult | Ambient | 20230711 | 27.7 | 36.29 | -63.3 | 8.09 | 30 | NA | JA | Temperature taken w/ YSI |
| 8/9/23 | 18:30 | Basin 1 | Ambient | 20230711 | 27.8 | 36.31 | -63.3 | 8.1 | 24 | NA | JA | Temperature taken w/ YSI |
| 8/9/23 | 18:30 | Basin 2 | High | 20230711 | 30.2 | 36.32 | -60.3 | 8.04 | 14 | NA | JA | Temperature taken w/ YSI |
| 8/9/23 | 18:30 | Basin 3 | Ambient | 20230711 | 28 | 36.26 | -62.5 | 8.08 | 14 | NA | JA | Temperature taken w/ YSI |
| 8/9/23 | 18:30 | Basin 4 | High | 20230711 | 30.3 | 36.32 | -61.7 | 8.07 | 11 | NA | JA | Temperature taken w/ YSI |
20230810
Checked on the corals at 6am, but no signs of spawning. If they had spawned, I’d expect to see eggs in the water and for the water to be cloudy from the sperm.
The pumps came back on last night around 9:30pm so the corals were not without pumping water for long. Prep work is mostly done for the spawning so there wasn’t a ton to do today. Hollie determined that we need to correct for immersion effect (ie putting the Apogee underwater) for the BIOS Apogee (model SQ-500), so I need to correct my PAR readings by multiplying the readings by 1.25. Read more about the correct factor here.
We tented the corals around 18:45 and watched until 2100. No spawning tonight unfortunately. I haven’t seen any Dlab spawning posts on Facebook or Twitter yet, so it doesn’t look like any Dlab in the Caribbean has spawned in August yet.
Here are the daily measurements from today:
| date | time | tank | treatment | tris.date | temp.C | sal.psu | pH.mV | pH.nbs | par | par_adj | flow.mL.5s | initials | notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8/7/23 | 10:00 | Adult | Ambient | 20230711 | 27.8 | 36.09 | -58.6 | 8.02 | 120 | 150 | NA | JA | Temperature taken w/ YSI; need to apply underwater corrections for light values |
| 8/7/23 | 10:00 | Basin 2 | High | 20230711 | 30.3 | 36.08 | -59.5 | 8.02 | 203 | 253.75 | NA | JA | Temperature taken w/ YSI; need to apply underwater corrections for light values |
| 8/7/23 | 10:00 | Basin 1 | Ambient | 20230711 | 27.8 | 36.08 | -60.3 | 8.03 | 230 | 287.5 | NA | JA | Temperature taken w/ YSI; need to apply underwater corrections for light values |
| 8/7/23 | 10:00 | Basin 4 | High | 20230711 | 30.6 | 36.09 | -59.6 | 8.02 | 114 | 142.5 | NA | JA | Temperature taken w/ YSI; need to apply underwater corrections for light values |
| 8/7/23 | 10:00 | Basin 3 | Ambient | 20230711 | 28 | 36.1 | -60.6 | 8.05 | 131 | 163.75 | NA | JA | Temperature taken w/ YSI; need to apply underwater corrections for light values |
| 8/7/23 | 13:30 | Adult | Ambient | 20230711 | NA | NA | NA | NA | 462 | 577.5 | NA | JA | Temperature taken w/ YSI; need to apply underwater corrections for light values |
| 8/7/23 | 13:30 | Basin 1 | Ambient | 20230711 | 27.7 | 36.12 | -59.5 | 8.03 | 698 | 872.5 | NA | JA | Temperature taken w/ YSI; need to apply underwater corrections for light values |
| 8/7/23 | 13:30 | Basin 2 | High | 20230711 | 30.4 | 36.13 | -58.4 | 8.03 | 665 | 831.25 | NA | JA | Temperature taken w/ YSI; need to apply underwater corrections for light values |
| 8/7/23 | 13:30 | Basin 3 | Ambient | 20230711 | 27.5 | 36.11 | -60.4 | 8.05 | 451 | 563.75 | NA | JA | Temperature taken w/ YSI; need to apply underwater corrections for light values |
| 8/7/23 | 13:30 | Basin 4 | High | 20230711 | 30.5 | 36.12 | -61.3 | 8.05 | 368 | 460 | NA | JA | Temperature taken w/ YSI; need to apply underwater corrections for light values |
I calculated par adjusted by multiplying the par column by 1.25 in Excel.
20230811
Checked on the corals at 6am, but no signs of spawning.
In the afternoon, we moved the adults to the smaller tanks and drained the larger tank to clean it, as it was getting pretty dirty and there was some algae build up. The corals were moved back after the tank was refilled. I also increased the temperature in the adult tank to 29°C to be closer to ambient in situ temperatures.
I read out the loggers from the tanks today. There were some issues reading out loggers Dlab-1 and Dlab 2 (logger metadata here). The part where the loggers connect to the base station was scratched so the loggers couldn’t be read out. I had to take them apart to read them out, but Dlab-1 appears to be completely broken. I need to replace that one.
Here is the logger data plotted by treatment:

The ambient increases at the end because we drained the tank and the loggers were exposed to the air for a period of time.
Here is the logger data plotted by tank:

Basin 2 and 4 are the high treatment tanks. Temperature in Basin 2 is slightly higher than Basin 4 and has more variability. We increased the temperature in Basin 4 by 0.4°F to try to match Basin 4 to Basin 2 a bit better.
We tented the corals around 18:30 and watched until 2200. No spawning tonight unfortunately. I haven’t seen any Dlab spawning posts on Facebook or Twitter yet, so it doesn’t look like any Dlab in the Caribbean has spawned in August yet. Similarly to the past few nights, we turned off the incoming water and turned the pumps back on. That way, if the corals do spawn overnight, the eggs/larvae will just be recirculating through the chiller, pump and tank instead of getting flushed out of the outflow pipe.
20230812
Checked on the corals at 6am, but no signs of spawning.




